Problem Set 1 - James Lab - University of California, San Francisco
advertisement

CHEMISTRY 112
School of Pharmacy
University of California, San Francisco
Problem Set I
Questions and Problems on Order, Molecularity and Rate Laws
The purpose of this exercise set is to help you to gain a thorough grasp of the concepts of order and
molecularity as applied in chemical kinetics and to give you practice in writing rate laws for elementary
and complex reactions. Answering the questions will help you to see if you possess the basic conceptual
knowledge related to the material we have covered in lecture. The first two problems are relatively easy
and designed to help you build confidence in your ability to set up mechanism problems and work them
through. Problem 3 is slightly more complex. All of these problems require use of the steady state
approximation. The fourth problem leads you to the derivation of one of the most important rate laws in
enzyme kinetics, the Michaelis-Menten equation; it uses a slight “twist”, as compared to the first three
problems. The fifth problem asks you to analyze some kinetic data using a form of the Michaelis-Menton
equation known as the Lineweaver-Burk equation. At the end of the set, you will find a old examination
problem that will make you think a bit harder than the first five problems and provide an opportunity to see
how your problem solving ability stacks up with that expected in test situations.
I. Questions
1.
2.
3.
4.
5.
Define an elementary reaction. Distinguish elementary reactions from complex reactions.
Define a zero order reaction; a first order reaction; a third order reaction.
Define a unimolecular reaction; a termolecular reaction.
An elementary reaction is oberved to be bimolecular. What can you say about its rate law?
A reaction is observed to be second order. Under what circumstances can you say something about its
molecularity?
6. Distinguish between molecularity and order of a reaction.
7. Certain reactions possess the following rate laws. If possible, give the overall order of the reactions in
each of the rate laws given below.
(a) d[A]/dt = -k[A]3/2
d[B]/dt = -k[A][B]/[C]1/2
(c) d[C]/dt = k1 [A][B]/{1 + k 2[B]}
(b)
8. Give, in words, the meaning of the following rate law: d[A]/dt = -k[A][B]
9. An elementary reaction proceeds according to the stoichiometric equation A + 2B Æ C.
Write the rate law for this reaction. Could you write a meaningful rate law for this reaction if you
weren't given the fact that the reaction is elementary?
10. Why aren't rate laws of the type d[A]/dt = -k[A]7 observed for elementary reactions?
II. Problems
1. The reaction sequence for formation of NO2 from NO and O2 has been experimentally determined to be
k1
NO + O2
NO3
k -1
NO3 + NO
k2
2 NO2
Assuming that the second reaction is very slow, determine the rate law that would be observed for this
complex reaction sequence.
2. Derive the rate equation for the following gas phase reaction sequence.
k1
A
B
k -1
B + C
k2
D
If the concentration of B is small compared with the concentration of A, C and D, then the steady state
approximation may be applied. Show that the resultant rate law is first order at high pressures and
second order at low pressures. What is the slowest step in each case? (Hint: Is there a relationship
between pressure and concentration in ideal gas systems?)
3. The reaction of A, B, and D to form products E, F, and G has been studied and the following mechanism
has been proposed.
k1
A
+ B
C
k -1
k2
C
+ D
E
C
k3
F
k4
C
G
(a) Using the steady state approximation, derive the rate law describing the rate of formation of E.
(b) The experimental rate law for the reaction was found to be second order overall. Under what
conditions will your rate law be second order overall?
(c) Assume that the reaction has been determined to be third order overall. Under what conditions could
your rate law be made to become third order overall?
(d) What is the overall order of the rate law you derived in (a) above?
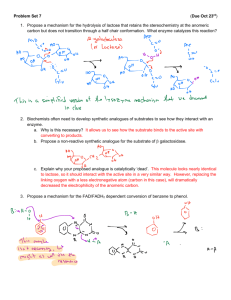
4. The simplest enzymatic reactions go according to the sequence
k1
k2
E
+
S
ES
E
+
P
k -1
where E is the enzyme, S is the substrate and ES is the enzyme-substrate complex. (a) Write out the rate
laws for the disappearance of substrate and enzyme-substrate complex and for the appearance of the
product. (b) Assuming that the steady state approximation can be applied to the enzyme-substrate
complex, write an expression for the steady state concentration of ES. (c) Noting that [E] 0 = [E] + [ES]
at any time (enzymes are catalysts and do not disappear during the reaction), show that the initial rate of
appearance of product P can be written as d[P]/dt = Vmax/{1 + (K M/[S])} where Vmax = k2 [E]0 and the
quantity KM = {k 2 + k-1}/k1 . This is the famous Michaelis-Menten equation, one of the fundamental
equations of enzyme kinetics. The quantity KM is known as the Michaelis constant. (d) show that if [S]
is much greater than KM, then the rate equation given above will be first order in enzyme concentration
(psuedo zero order overall) and that if [S] is much less than KM, then the rate equation will be first order
in both substrate concentration and in enzyme concentration (psuedo first order overall). (e) Show that
Vmax is the maximum rate of product formation.
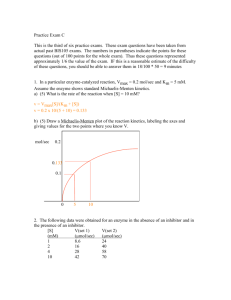
5. The initial rate of oxidation of sodium succinate, by dissolved oxygen, to form sodium fumerate in the
presence of the enzyme succinoxidase can be represented by the Michaelis-Menten equation derived in
the previous problem. The following experimental data were taken, where the subscript 0 refers to a
measurement made near time = 0:
103 ¥ [S], M
106 ¥ d[P]/dt}0 , M s-1
10.0
1.17
2.0
1.0
0.5
0.33
0.99
0.79
0.62
0.50
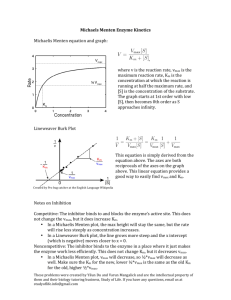
Note that if we invert the Michaelis-Menten equation, we obtain:
1/v0 = 1/(d[P]/dt)0 = 1/Vmax + KM/(Vmax[S])
and hence, by plotting 1/v0 versus 1/[S], we can obtain values for Vmax and KM. This plot is known as a
Lineweaver-Burk plot and is useful in studying the enzymatic degradation of drugs. Plot the data and
obtain values for Vmax and KM. (In using the above data, remember that if 103 ¥ y = 1, then y = 0.001.)
III. Problem from an Old Examination
1. (Midterm, 1999-2000) Compound A reacts to form the products C, D and E in the
presence of M. This reaction is thought to go according to the following mechanism:
k1
A
+
M
3B + M
k-1
k
3B 2
C
k
3B 3
D
k
3B 4
2E
(a) (5) Identify the intermediate(s) in this reaction
(b) (5) Is there a catalyst entering into this proposed mechanism? Why or why not?
(c) (15) Derive a rate law for formation of D, assuming that the steady state approximation
can be applied to any intermediate(s) in the above mechanism.
(d) (5) Under what conditions would the rate law you derived in part (c) be second order
overall?
(e) (5) Under what conditions would the rate law you obtained in part (c) be first order
order overall?
(f) (5) What is the rate law for formation of the product E?