Chemical Bonding Guided Notes
advertisement

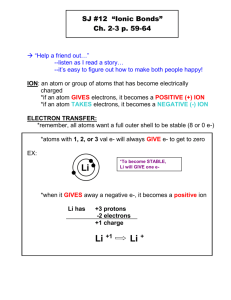
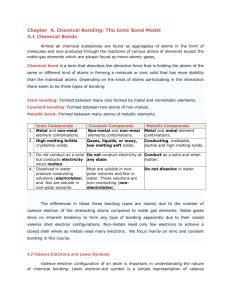
Name ____________________________________________ Date _______________ Physical Science Period ________ Chapter 6: Chemical Bonding Guided Notes I. Why do Atoms Combine? A. Chemical Formula 1. The chemical formula shows: a. The ___________________________ in the compound. b. The ratio of their ______________________________. _________ H2O ____________ B. Chemical Bond 1. Strong attractive force between _____________ or ions in a molecule or compound. 2. Formed by: a. _______________________ electrons, (e-) by losing or gaining electrons. b. _______________________ electrons (e-) C. Stability 1. Octet Rule a. Most atoms form bonds in order to have ______ valence electrons (e-). b. _______________ outer energy level. c. Want to be like the Noble Gases (group 18). 2. Stability is the driving force behind bond formation. All atoms bond to become stable. 3. Transferring e- Ne 4. Sharing e- II. Kinds of Chemical Bonds A. Ionic Bond 1. Attraction between 2 __________________________ charged ions. 2. Ions are _____________________ atoms. 3. A _____________________ is a ______ charged ion. Forms when an atom loses an electron. Formed by ___________________. 4. A _____________________ is a ______ charged ion. Forms when an atom gains an electron. Formed by ___________________. 5. Formed by _______________________ electrons (e-) from a _____________ to a ______________. 6. Ions form a 3-D crystal lattice. B. Covalent Bond 1. Attraction between neutral atoms. a. Formed by ____________________________ electrons between two nonmetals. b. Covalent bonds result in molecules. Cl2 NH3 H2O 2. ________________________________ Covalent Bond a. Electrons (e-) are _____________________________ equally. b. Usually identical atoms. 3. ________________________________ Covalent Bond a. Electrons (e-) are shared ______________________ between 2 different atoms. b. Results in partial opposite charges. C. Comparison Chart Ionic Covalent Electrons Melting Point Soluble in Water Conduct Electricity Other Properties Types of Elements D. ____________________ types of bonds Some compounds have a mixture of ______________ and __________________ bonds. These generally contain POLYATOMIC IONS. _____________________ ____________ are groups of non-metal elements bonded. _____________ together that have an overall ________________. Generally polyatomic ions will form an ionic bond with metal. They are easy to recognize because they have ______________ or more elements in the compound. Examples: ______________________________, __________________________ E. Practice: What type of compound is shown or described below? 1. NaCl ________________________________________ 2. CO2 ________________________________________ 3. H2O ________________________________________ 4. Fe2O3 ________________________________________ 5. Ga(C2H3O2)3 ________________________________________ 6. High melting point ________________________________________ 7. Liquid or Gas ________________________________________ 8. Doesn’t dissolve in water ________________________________________ III. Naming Molecular (Covalent) Compounds A. Molecular Names 1. ____________________________________________________________________ 2. ____________________________________________________________________ 3. ____________________________________________________________________ 4. ____________________________________________________________________ Prefix Examples: CCl4 ____________________________ Subscript 1 2 3 4 5 Prefix Subscript 6 7 8 9 10 N2O _____________________________ SF6 ______________________________ B. Molecular Formulas 1. Write the _____________________ metallic element first. 2. Add subscripts according to prefixes. Examples: phosphorus trichloride dinitrogenpentoxide ___________________ ___________________ dihydrogen monoxide ___________________ 3. The seven Diatomic elements are: Br2 I2 N2 Cl2 H2 O2 ***In nature these elements are never alone!!*** IV. F2 Naming Ionic Compounds A. Oxidation Number 1. The charge on an ion. 2. Indicates the # of electrons (e-) gained/lost to become ________________________. Oxidation Chart Group # Valence eOxidation # 1 1 2 2 13 3 14 4 15 5 16 6 17 7 18 8 B. Ionic Names 1. __________________________________________________________________________________ 2. __________________________________________________________________________________ 3. __________________________________________________________________________________ Examples: NaBr ___________________________ Na2CO3 SnCl4 ______________________________ _____________________________________ C. Ionic Formulas 1. Write each ion. Put the _______________ first. 2. Overall charge must equal zero. a. If charges _______________, just write the symbol. b. If not, crisscross the _______________ to find subscripts. 3. Use parentheses when more than one ______________________________ is needed. 4. Roman _______________ indicate the oxidation number (oxidation #). D. Writing Ionic Formulas 1. Write each ion. Put the cation first. 2. Overall charge must equal zero. 3. If charges cancel, just write the symbols. 4. If not, crisscross the charges to find subscripts. 5. Use parentheses when more than one polyatomic ion is needed. E. Writing Ionic Formulas – Crisscross method 1. IF the charges don’t balance the number of the charge becomes the subscript for the opposite element or polyatomic ions. 2. Example 1: Mg +2 N-3 _______________________ The charges BALANCE thus disappear Example 2: Al+3 SO4-2 _______________________ Remember to use parentheses when more than one polyatomic ion is needed. Example 3: Na+1 C2H3O2-1 _______________________ Remember If charges cancel, just write the symbols F. Writing Ionic Formulas Examples: potassium chloride magnesium nitrate _______________________ ______________________ aluminum oxide __________________________