Chemical Names & Formulas: Chemistry Study Guide
advertisement

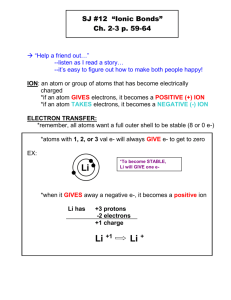
Chemical Names and Formulas Key Vocabulary Monoatomic Ion – Ions formed from a single atom Binary compound – Compounds composed of two elements Nomenclature – the “naming system” of binary ionic compounds Oxyanion – polyatomic ions that contain oxygen Salt – an ionic compound composed of a cation and the anion from an acid Chemical Formulas Subscripts after an elemental symbol represent the number of atoms of the element in the compound Molecular formulas represent how many atoms of each element are in a compound. (C2H8, H2O, C6H12O6) Ionic formulas show the simplest ratio, cations to anions, of atoms in the lattice Parenthesis are used around a polyatomic ion in a chemical formula Examples Al2(SO4)3, Cu(PO4)2, Sr(NO3)2 Monoatomic Ions – Ions formed from a single atom Group 1 elements may lose one electron = 1+ Group 2 elements may lose two electrons = 2+ Group 13 elements may lose three electrons = 3+ Group 17 elements may gain one electron = 1Group 16 elements may gain two electrons = 2Group 15 elements may gain three electrons = 3Transition metals have various possibilities (no pattern) Naming monatomic Ions Monatomic cations are simply called by the element’s name *the stock system is used when there is more than one possibility for an element (more on this later) Monatomic anions end in IDE such as Fluorine – Fluoride, Oxygen – Oxide (reference table 1 on page 221) Binary Ionic Compounds 1. Write the symbols for the ions with the cation first (Al3+ O2-) 2. Cross over the charges writing the charge of one ion as the subscript for the other ion (Al23+ O32-) 3. Multiply subscript and superscript for each ion then find the lowest whole number ratio (Al2O3) Name it!!! Aluminum Oxide Examples: Zn2+ IZn2+ S2(More on p 223 practice problems) Stock system of Nomenclature Sometimes elements, usually transition metals, can form different cations. The stock system was developed to show their different charges by using roman numerals (Refer back to page 221) Examples Cu (I) or Cu (II), Sn(II) or Sn(IV), V(II) or V(III) or even V(IV) The same method is used as for binary ionic compounds Cr3+ F- combines to form CrF3 and is called Chromium (III) Fluoride Oxyanions and other polyatomic ions Elements can bond together with different numbers of oxygen atoms (oxyanions) If there are 2 possibilities: greater number of oxygen atoms ends with –ate, smaller number of oxygen atoms ends with – ite. If there are more possibilities use Hypo- for a prefix when there are less and use Per- when there are more. Examples: Sulfite or Sulfate Nitrite or Nitrate Hypochlorite, chlorite, chlorate, perchlorate AgNO2 or AgNO3 Naming Binary Molecular Compounds There are two systems the newer system is the stock system previously discussed. The older system uses prefixes to denote how many atoms of an element are present. Number Prefix Number Prefix 1 Mono 6 Hexa 2 Di 7 Hepta 3 Tri 8 Octa 4 Tetra 9 Nona 5 Penta 10 Deca General Rules: Write the element with the lesser number of atoms first Drop the last vowel of the prefix if the element starts with a vowel Placing an –ide after the second element usually means there are 2 elements Acids and Salts An acid refers to a water based solution that has unique properties. Binary acids are composed of two elements (Hydrogen and something else) Oxyacids consist of hydrogen, oxygen, and some other element(s) If a hydrogen is lost from an acid an anion is usually produced A salt is formed if the anion from an acid combines with a cation Resources Modern Chemistry text (Holt, Rinehart, and Winston) Page 219 - 231