Summary of Reactions -ws
advertisement

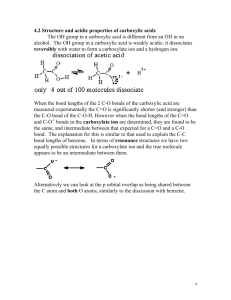
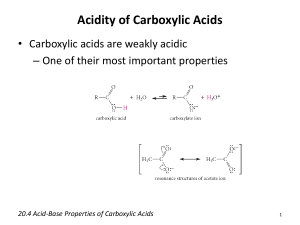
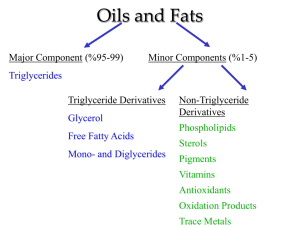
Name_________________________________________________________ Period _________ Date ________ Summary of Reactions Oxidation, Neutralization, Esterification & Saponification Oxidation: 1. Draw the structure of the carboxylic acid produced by the oxidation of propanol 2. Write the reaction for the production of butyric acid from an alcohol. 3. Completely oxidize 2-methylpentanol to a carboxylic acid. Name the products. Neutralization: 1. What two products are formed by the neutralization of a carboxylic acid? 2. Write the reaction for the neutralization of benzoic acid with potassium hydroxide. Name the products. 3. Write the reaction for the neutralization of propanoic acid with sodium hydroxide. Name the products. Esterification: 1. Complete the following reaction: Butanoic acid + methanol H+ water + _____________________________ 2. Write an equation (using structures AND names) showing the formation of propyl benzoate. 3. What two molecules are needed to produce ethyl acetate? Draw and name the molecules. 4. Draw the structures of the acid and alcohol needed to produce water and: Saponification: 1. Saponification is the _______________ hydrolysis of an __________________ to produce a __________________________________________ and ______________________. 2. Write the reaction for the saponification of propyl-2-methyl butanoate using an aqueous solution of potassium hydroxide. Name the products. 3. Write the reaction for the saponification of 2-bromoethyl salicylate using an aqueous solution of sodium hydroxide. Name the products. Matching – some answers are used more than once: 1. _____ Long chain carboxylic acids a) esters 2. _____ Fatty acid salt b) soap and alcohol 3. _____ Known for its scents c) salt and water 4. _____ Products of neutralization d) ester and water 5. _____ Products of esterification e) fatty acids 6. _____ Products of saponification f) carboxylic acid 7. _____ Product of oxidation of an aldehyde g) soap 8. _____ Carboxylic acid + base 9. _____ Ester + base 10. _____ Carboxylic acid + alcohol 11. Write an equation showing the formation of methyl acetate using only alcohols.