Aspirin Synthesis
advertisement

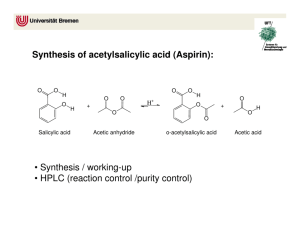
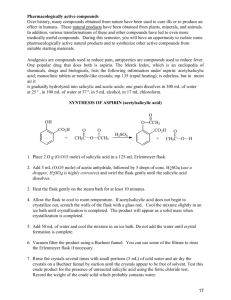
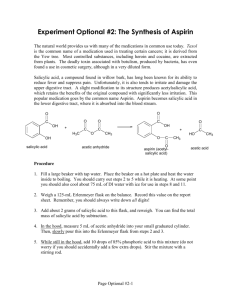
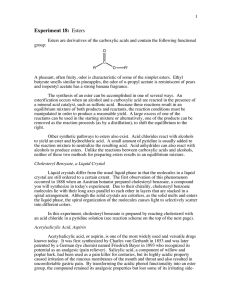
Lab 5: Synthesis of Aspirin Objectives: - To synthesize aspirin and better understand carbonyl chemistry. - To analyze the aspirin for impurities. Introduction: Aspirin (acetylsalicylic acid) can be prepared by the reaction between salicylic acid and acetic anhydride: O OH O OH O O H+ OH O O + + O O HO In this reaction, the hydroxyl group (-OH) on the benzene ring in salicylic acid reacts with acetic anhydride to form an ester functional group. Thus the formation of acetylsalicylic acid is referred to as an esterification reaction. This reaction requires the presence of an acid catalyst, indicated by the H+ above the equilibrium arrows. When the reaction is complete, some unreacted salicylic acid and acetic anhydride will be present, along with acetylsalicylic acid, acetic acid and the catalyst. Crystallization will be used to purify the acetylsalicylic acid. The purification process is facilitated by the addition of water after the crystals have formed. The water decreases the solubility of acetylsalicylic acid and dissolves some of the impurities. The most likely impurity in the final product is salicylic acid itself, which can arise from incomplete reaction of the starting materials or from hydrolysis (reaction with water) of the product during the isolation steps. The hydrolysis reaction of acetylsalicylic acid produces salicylic acid. Salicylic acid and other compounds that contain a hydroxyl group on the benzene ring are referred to as phenols. Phenols form a highly colored complex with ferric chloride (Fe3+ ion). Aspirin is not a phenol because it does not possess a hydroxyl group has been converted to an ester. Because aspirin will not give the color reaction with ferric chloride, the presence of salicylic acid in the final product is easily detected. The purity of your product will also be determined by obtaining the melting point. Pre Lab Questions: (To be submitted at the beginning of the lab period) 1. Summary of the procedure in your own words. 2. What temperature is the reaction run at and why is it heated? 3. Why is the mass of product determined on a different day than the reaction is run? 4. What type of melting point would you expect from an impure product? Edited by Nick Buker 02/10/10 1 Procedure: 1. Prepare a hot water bath using a 250-mL beaker and a hot plate. Use about 100 mL of water and adjust the temperature to about 50°C. 2. While the bath is coming up to temperature, weigh 1.10 g salicylic acid (MW = 138.1) and place this in a 25-mL Erlenmeyer flask. Record the actual weight on the Report sheet. 3. Use a graduated cylinder to obtain 2.5 mL of acetic anhydride (MW = 102.1, density = 1.08 g/mL) and add this to the flask. Then add 4 drops of concentrated phosphoric acid. Use caution with both the acetic anhydride and the phosphoric acid. Also add a magnetic stir bar to the flask. 4. Once the bath has come up to temperature, clamp the flask so that the flask is partially submerged in the hot water bath. Stir the mixture with the magnetic stir bar until the salicylic acid dissolves. Heat the mixture for another 10-12 minutes after the solid dissolves to complete the reaction. 5. Remove the flask from the water bath. Remove the stir bar with a forceps or by using a larger magnet on the outside of the flask. 6. Allow the flask to cool to room temperature during which time the acetylsalicylic acid should begin to crystallize from the reaction mixture. If it does not crystallize, scratch the bottom of the flask with a glass rod. 7. Cool the mixture in an ice-water bath for several minutes to yield more crystals. 8. After crystal formation is complete (usually when the product appears as a solid mass), add 10 mL of water and stir with a glass rod. 9. Collect the product by vacuum filtration on a Buchner funnel. When you have removed as much product as possible for the flask, add several mL of cold water to the flask, swirl the flask, and transfer the remaining crystals and the water to the Buchner funnel. 10. When all the crystals have been collected in the funnel, rinse them with several 2mL portions of cold water. 11. Continue drawing air through the crystals on the Buchner funnel by suction until the crystals are nearly dry (approximately 5 minutes). Remove the top of the funnel and allow the crystals dry overnight. 12. When you return to lab another day, weigh the dry product and calculate the percentage yield of acetylsalicylic acid (MW = 180.2). Determine the melting point of the product. The literature melting point is 135-136 °C. You will also be running the ferric chloride test for purity. Edited by Nick Buker 02/10/10 2 Ferric chloride test for purity When you return to the lab, determine if there is any salicylic acid remaining in your product by carrying out the following procedure. Obtain three small test tubes. Add 0.5 mL of water to each test tube. Dissolve a small amount (about 0.05g) of salicylic acid in the first tube. Add a similar amount of your product to the second tube. The third test tube, which containing only solvent, will serve as the control. Add one drop of 1% ferric chloride solution to each tube and note the color after shaking. Formation of an ironphenol complex with Fe(III) gives a definite color ranging from red to violet, depending on the particular phenol present. Lab Report Guide: - 1. Results (3 pts) o Calculate the percentage yield (show work, including calculating the limiting reagent). o Generate a table summarizing the result of the melting point and ferric chloride tests. - 2. Error Analysis (2 pts) o Typed discussion of measured melting point vs literature melting point and description what these result mean. - 3. Post Lab Questions (5 pts) o Typed answers to the Post Lab questions. Note that single sentence answers will not suffice. State the answer to the question followed by a brief description of the evidence supporting that answer. Post Lab Questions: 1. Give three reasons why the % yield was less than 100%. Each reason should be specific to our procedure. For example, product left on glassware is not specific enough because it applies to almost procedure. Give reasons specific to this technique. 2. Describe the color, shape and size of the crystals formed. 3. Why do impure materials tend to melt at lower temperatures than pure ones? 4. If you were to repeat this experiment, what would you do differently? 5. Salicylic acid contains two acidic groups: a carboxylic acid and a phenol. Why might it be desirable to do away with one of these groups by converting salicylic acid into aspirin? Edited by Nick Buker 02/10/10 3