Test 1
advertisement

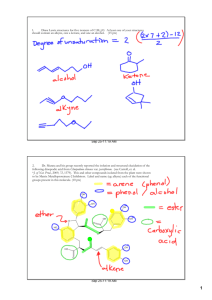
C 202 Organic II Test 1 February 8th, 2007 I. Identify (2 pts each) Markovnikov’s rule, homolysis, radical, hydrogen abstraction, propagation step, Regioselective reaction, Stereospecific reacion, co-polymer, syndiotactic, isotactic, atactic, II. Polymer Chart. Fill in each open cell. (1 pts each) Monomer Polymer Names Polystyrene n Cl Cl Cl PVC n Polyacrylonitrile NC F F F F F F F F F F F F F F n F F COOCH3 COOCH3 COOCH3 H 3C CH3 CH3 Poly (methylmethacrylate) n III. Nomenclature (3 pts each) A. Name the following: OH Cl B) Give the structure of (2S)-2-Chlorohex-3-yn-1-ol IV. Reactions (3 pts each) Provide the product(s) for the reactions below: NBS A) D) C H3 CH2CH 2C Cl B) CH3 H CH2 CH3 Br2 G) 1) OsO 4 2) NaSO 3 / H2 O 1) O3 E) 2) Zn / HOAc H) 1) O3 2) Zn / HOAc HBr C) 1) KMnO 4, -OH heat F) ROOR 2) H3 O + I) Br2 H2 O 1) BHtotal) 3 :T HF V. Mechanisms (12 pts A) (8 pts) Provide the product(s) for the below and the mechanisms that account for 2 ) H2reactions O2 , -O H each product. A)B) (4 pts) + Br2 B)C) (8 pts) Provide a mechanism for the polymerization of ethene. Be sure to include and label special cases such as combination, disproportionation, and backbiting in the appropriate steps. VI. Synthesis (10 pts total) A) Provide a retrosynthetic pathway for the synthesis of 2-bromobutane from compounds of 2 carbon atoms or less. (5 points) B) Provide a retrosynthetic pathway for the synthesis of 1,2-pentandiol from compounds of 2 carbon atoms or less. (5 points) VII. Essay. (10 pts) Write a brief essay describing polymerizations including such terms as: Chaingrowth/addition polymers, Cationic polymerizations, Anionic polymerizations, copolymer, atactic, syndiotactic, and isotactic. You should also include examples. VIII. NMR/MS (15 points) A. The compound CH3F, gave peaks in a mass spectrum at 14, 15, 19, 31, 32, 33, 34, 35 with the following relative intensities 17.2, 100, 2.0, 10.4, 9.4, 89.5, 95.4, and 1.1 respectively. Provide a structure for each of the ions associated with those peaks and show how they were formed. (the peak at 34 is the molecular ion peak) (7 points) B. Using the following Molecular formulas, NMR data, and/or IR data, provide the structure of the molecule. i. C4H8O NMR: triplet at 1.05 (3H), singlet at 2.13 (3H), quartet at 2.47 (2H). IR: Stong peak near 1720 cm-1 (4 points) ii. C4H7BrO2 NMR: triplet at 1.25 (3H), multiplet at 2.07 (2H), triplet at 4.23 (1H), singlet at 10.97 (1H). IR: broad peak in 2500-3000 cm-1 region and a peak at 1715 cm-1 (6 points)