Exam 1
advertisement

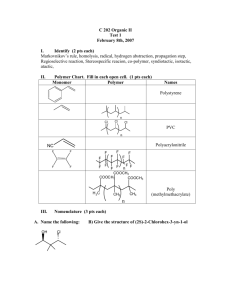
Spring 2013 Chem 302 Houseknecht Name_____________________________________ I _______________________________________ (sign name) affirm that my work upholds the highest standards of honesty and academic integrity at Wittenberg, and that I have neither given nor received any unauthorized assistance. Exam 1 – February 1, 2013 There are 6 pages (including this one) and 9 questions on this exam. Read each question carefully before beginning. Show work for partial credit. A periodic table is attached to the end of this exam. You may remove it and use it for scrap paper. You may also use the lower portion of this cover page as scrap paper. If you would like any more scrap paper please ask. Good luck. Spectra on this exam have been obtained from the SDBS website or simulated, (http://www.aist.go.jp/RIODB/SDBS). Exact Masses Hydrogen – 1.00783 Carbon – 12.00000 Nitrogen – 14.00307 Oxygen – 15.99491 Alkenes in fingerprint region Monosubstituted – 1000 and 900 cm-1 Disubstituted Cis – 750-650 cm-1 Trans – 950 cm-1 Trisubstituted – 850-790 cm-1 (medium) Aromatics in fingerprint region Monosubstituted – 770-730 cm-1 AND 710-690 cm-1 Ortho (1,2) – 770-735 cm-1 Meta (1,3) – 850-810 cm-1 AND 710-690 cm-1 Para (1,4) – 840-810 cm-1 Typical coupling constants Alkenes cis ~ 6-12 Hz trans ~ 12-18 Hz gem ~ 0-3 Hz allylic ~ 4-10 Hz Aromatics ortho (1,2) ~ 6-8 Hz meta (1,3) ~ 1-3 Hz para (1,4) ~ 0-1 Hz Spring 2013 Chem 302 Houseknecht 1. (10 pts) Answer the following multiple choice questions. Show thought process for partial credit. a. Which of the following peaks are expected in the MS of 2-methylbutan-2-ol? Circle all correct answers. i. 86 ii. 73 iii. 59 iv. 29 b. Cortisone is a molecule containing only C, H, and O with eight degrees of unsaturation and an exact mass of 360.1937. What is its molecular formula? i. C24H24O 3 iii. C21H28O5 ii. C23H20O 4 iv. C20H24O 6 c. Which of the following bond types has the greatest intensity in infrared spectroscopy? i. C=O stretch iii. C-C stretch ii. C=C stretch iv. C-H stretch d. Predict the number of carbon resonances you would expect in the 13C NMR spectra of methylcyclopentane. i. 2 ii. 4 iii. 5 iv. 6 e. The frequency of light absorbed by a 1H nucleus in NMR is increased by which of the following. Circle all correct answers. i. Nearby, non-equivalent nuclei in the spin state ii. Increased electron density iii. Nearby electronegative atoms iv. Increased applied magnetic field, Bo 2. (12 pts) Provide a structure for three of the peaks in the MS of heptan-3-one below. m/z 27.0 28.0 29.0 39.0 41.0 43.0 57.0 71.0 72.0 85.0 114.0 Int 22.8 8.2 79.1 8.8 33.7 15.2 100.0 5.6 32.9 43.3 14.0 Spring 2013 Chem 302 Houseknecht 3. (12 pts) Predict three important peaks in the IR spectra of the ester below and the specific bond vibrational responsible for each. 4. (4 pts) Explain why the C=O stretch of pent-3-en-2-one occurs at a lower frequency than that of pent-4-en-2-one. 5. (12 pts) Predict the multiplicity and chemical shift of each type of proton in isopropyl propanoate. Label each for clarity. Spring 2013 Chem 302 Houseknecht 6. (12 pts) The HC region of the 1H NMR spectra of bromobenzene is shown below. Draw a tree diagram that accounts for the observed multiplicity of HC and calculate the JAC and JBC values in Hz. Show calculations for complete credit. 7. (12 pts) Provide the structure of the molecule that has the mass and 13C NMR spectra shown below. Show work for complete credit. m/z 27.0 39.0 41.0 42.0 43.0 122.0 124.0 Int 25.6 9.0 31.0 7.7 100.0 8.6 8.3 Spring 2013 Chem 302 Houseknecht 8. (14 pts) Provide the structure of the molecule that has the spectra shown below. Show work for complete credit. 3H, s 3H, s 3H, t 2H, q s Spring 2013 Chem 302 Houseknecht 9. (12 pts) Provide the structure of the C5H10O molecule that has the 1H NMR spectra shown below. Show work for complete credit. 3H, s 3H, t 2H, t 2H, sextet Extra Credit (5 pts) Explain why the protons at C1 of but-1-ene are spin-spin coupled to one another with a coupling constant of 2.5 Hz, but those of 2-methylpropene are not spin-spin coupled.