Answers, PS3
advertisement

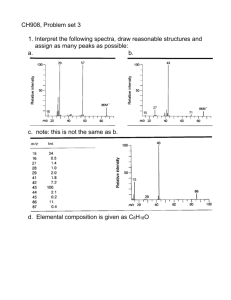
CH908, Problem set 3 1. Interpret the following spectra, draw reasonable structures and assign as many peaks as possible: a. b. Answer: 3-pentanone c. note: this is not the same as b. Answer: 2,3-butanedione. Answer: 3-methyl, 2-butanone d. Elemental composition is given as C8H18O Notes: alkane/alkene series. 31, 45 indicate an oxygen, probably not an alcohol, from the intensity. Something special about 57, indicating either a very stable neutral radical loss, or a very stable even electron product ion, or very high symmetry – so that this species can be formed several ways. Partial answer: C4H9-O-C4H9 several isomers are possible, but intense 41 suggests branching. 87 peak suggest the branch (on one side at least) is not at the first carbon. To determine exactly which isomer, it’d be necessary to have the whole series of isomer spectra. Answer: diisobutyl ether e. Notes: no nitrogens. methyls and ethyls and propyls are present. Even electron 58 ion is strange. 31, 45 indicates an oxygen, but intensity says it’s not an alcohol, probably not even an ether – probably carbonyl. Answer: 4-methyl, 2 pentanone. 58 peak comes from the McLafferty rearrangement. f. Same as above, but the McLafferty rearrangement even electron ion peak is heavily suppressed, and shifted by 14 Da to 72 Da. Also, 57 peak is much higher, probably C4H9+. Answer: 3-methyl, 2 pentanone. 2. A product ion mass or neutral loss of 91 Da indicates what moiety? Why? Draw the structures. C7H7•, Toluyl or tropylium (7 membered aromatic ring). 3. In EI ions, where are the charge site and the radical site located initially? Why? Do they stay together during fragmentation? First non-bonding electrons from heteroatoms, then pi bonding electrons, then sigma bonding electrons. This simply follows the energetics of electron binding. The charge site and radical site almost always move apart in the fragmentation because the electron-pushing rules are different for charges and radicals (hemolytic vs. heterolytic bond cleavage) 4. Define the terms 'distonic ion' and 'charge remote fragmentation'. Distonic ion: an radical ion where the charge and radical are at different positions. Charge remote fragmentation: fragmentation that is in a distant part of the molecule from the charge, usually caused by long-range radical rearrangements (hydrogen abstraction)