Mass Spectrometry
advertisement

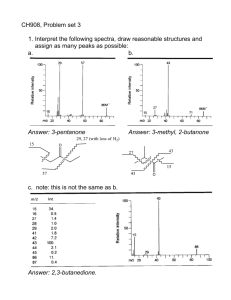
Mass Spectrometry Application of MS • Characterization of compound • Identification of unknown substance MS Instrument Mass Spectrum MS Library Databases Example: Orange Extract Molecular Ion Peak • • • • Determination of molecular mass Rule of Thirteen (Possible molecular formulae) Odd vs. Even molecular mass Isotopes – M + 1 peaks – M + 2 peaks • Nominal Mass vs. Exact Mass Empirical Formula and MS • What is the MF of a compound with the following data: M+= 110; %H = 12.72; %C = 87.27? • Answer: C8H14 Rule of 13 • What if you are only given M+ data? • Determine a range of possibilities using rule of 13 • Divide M+ by 13 – Quotient = #C – Quotient + remainder = #H – Replace CH4 with O, etc. • Example: Give three MF for M+ = 110 Odd Mass • Nitrogen rule Isotopes Remember: With MS, you are considering individual molecules, not “average” molecules! M+ + 1 Peak • Arises from sum of all possible isotopes • C-13 is most prevelant • Therefore, we can use M+1 to determine the likely number of carbon atoms in a molecule Apply to CH4 • In a group of CH4: – Most are 12C and 1H – About 1% are 13C and 1H – Small % are 12C and 2H – VERY SMALL % are 13C and 2H M+ = 16 amu M+1 = 17 amu M+1 = 17 amu M+2 = 18 amu • Application of M+ + 1 peak: determine #C = (M++1 intensity/M+ intensity)/(1.1/98.9) = (M+1 intesity/M intensity)/0.011 M+2 peak Examples MS Instrumentation • Mass analyzer – Magnetic sector – Quadrupole mass sorter – Time of Flight (TOF) HRMS • Nominal Mass • “Exact” Mass • Advantage of HRMS – Unique mass – Compare C8H14 with C7H10O – Nominal: 110 amu – Exact: 110.10962 vs 110.07320 MS Instrumentation • Ionization – EI – CI – ESI – FAB – MALDI Fragmentation • Unstable molecular ion: radical cation • Only cations observed – f+ (directly) – f . (indirectly) Mechanisms • Draw Lewis structure with electron knocked out of least stable place – Lone pair – Pi bond – Sigma bond • Fishhook arrow Example: Ethane Inductive Cleavage Example: Propane Typical Aliphatic Fragments: 15, 29, 43, 57, 71… Loss of Alkyl Fragments • • • • • 172-___=143 172-___=129 172-___=115 172-___=___ 172-___=___ Functional Groups • • • • • Benzylic group Alkenes Alcohols, amines, ethers, etc Carbonyls: alpha fragemntation Carbonyls: McLafferty fragmentation Benzylic Group • Weakest bond most stable ions/radicals • Rearrangements • Trypolium ion Alkenes • Allylic bond breaking • Resonance stabilized cations/radicals Amines/Alcohols/Ethers • Inductive cleavage • alpha bond fragmentation • Stabilized cation! Importance of alpha fragmentation Carbonyls: Alpha Fragmentation Carbonyls: McLafferty • Gamma abstraction • Even mass fragment • How would McLafferty fragmentation distinguish these ketones? Applying MS • What is the cause of the base peak? The parent peak? Identifying Fragments • What are the most likely identities of the ions that causes peaks at 41, 43, 69, and 84? • What causes the little peak above 84? • What is the molecular ion peak? Why is it not visible? • Why is the peak at 59 bigger than the peak at 73? • Draw a mechanism for the loss of neutral hydroxide radical. What is the ion peak visible from this fragmentation? Case Study • M+ = 114 and C=O known to be present – Nitrogen? – Cl/Br? – 72 suggests… • Strategy – Rule of 13 – Table of mass peaks and fragments Problem • Provide structure that matches this spectrum: Gas Chromatography/Mass Spec • Identifying components in mixtures • Separation then identification • GC-MS