CH 908: Mass Spectrometry Lecture 3
advertisement

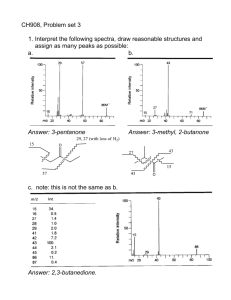
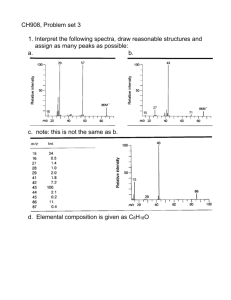
CH 908: Mass Spectrometry Lecture 3 Interpreting Electron Impact Mass Spectra – Continued… Recommended: Read chapters 5,8 of McLafferty Prof. Peter B. O’Connor Objectives for this lecture • Fragmentation pathways for radical molecular ions – Alpha cleavage – Inductive cleavage – Elimination – Rearrangements • Fragmentation series – – – – – Alkanes Aldehydes/Ketones Alcohols/sulfides amines esters/amides • Isomer differentiation • The McLafferty rearrangement Fragmentation Pathways Radical Site Initiation (a-Cleavage) - 1 This is particularly important for ions that contain N or O atoms. Electron pairing occurs by the transfer of an odd electron from the bond alpha to the atom carrying the charge and the transfer of the odd lone pair electron to form a new bond. The remaining electron from the alpha bond is lost on the radical that is eliminated as a result of the bond breaking. Radical Site Initiation (a-Cleavage) - 2 In this type of fragmentation, the charge site does not move but the radical site moves as a result of the alpha bond breaking. In the example below, C - X is the alpha bond broken. An example is the fragmentation of carbonyl compounds: C - X CO+ + X. || O+. (X = H, R, OH, OR, Cl, NH2) aldehydes, ketones, acids, esters, acid halides, amides Mechanisms of Alpha Cleavage X = H, R, OH, OR, Cl, NH2 for aldehydes, ketones, acids, esters, acid halides and amides. m/z 30 is the base peak in primary amine spectra Primary alcohols give the m/z 31, ethers often give m/z 45, 59 . . by this reaction The ease of loss of alkyl groups is R1>R2>R3 where this is also the order of decreasing size. C4H3+. C6H5+. [M-H.]+ M+. Charge Site Initiation (Inductive Cleavage) i-cleavage, C3H7+ α-cleavage, -C3H7• i-cleavage, C2H5+ α-cleavage, -C2H5• M+. -O• α-cleavage, -C2H5• α-cleavage, -NH2• M+. [M-16]+. characteristic of amides [CH2OH]+ -C2H4 from 84 [M-H2O]+. -CH3 M+. absent α-Cleavage, -C3H7 [CH2OH]+ i-Cleavage [C2H4OH]+ 3 examples of [CH2OR]+ ions at m/z 31, 59 and 87 ? α-Cleavage, -CH3 M+. [CH2NH2]+ base peak of primary amines M+. Loss of the Largest Alkyl Radical -1 Where there is a choice of alkyl radicals that can be lost in an alpha-cleavage, the largest radical is lost preferentially followed by the next largest, etc. Thus, the most intense peaks should be derived from the loss of the largest alkyl radical. Loss of the Largest Alkyl Radical - 2 The loss of H or an alkyl radical gives rise to the following series of ions for different classes of compounds Aldehydes and ketones m/z 29, 43, 57, 71 . .. Aldehydes usually give a fairly prominent m/z 29 peak (CHO+) and a weaker [M - 1]+ peak. Methyl ketones often give m/z 43 (CH3CO+) as the base peak. Loss of the Largest Alkyl Radical - 3 Alcohols and ethers m/z 31, 45, 59, 73 . . . Primary alcohols typically give a m/z 31 peak (CH2OH+), often of fairly low intensity. Methyl ethers give m/z 45 peaks, again often of low intensity (charge induced fragmentation usually predominates). Amines m/z 30, 44, 58, 72 . . . n-alkyl amines often give m/z 30 as the base peak whereas secondary and tertiary amines often give m/z 44 and 58 as base peaks together with m/z 30 C3H7 loss C2H5 loss CH3 loss M+. absent Loss of the largest alkyl radical [M-C5H11]+ [M-C3H7]+ [M-CH3]+ M+. Fragmentation Pathways Fragmentation: Alkanes Hexane shows the same fragmentation pattern as other unbranched alkanes. Alkyl carbocations at m/z=15, 29, 43 and 57 Da provide the dominant peaks in the spectrum. The m/z=57 butyl cation (M-29) is the base peak, and the m/z=43 and 29 ions are also abundant. Chain branching clearly influences the fragmentation of this isomeric hexane. The molecular ion at m/z=86 is weaker than that for hexane itself and the M-15 ion at m/z=71 is stronger. The m/z=57 ion is almost absent (try to find a simple cleavage that gives a butyl group). An isopropyl cation (m/z=43) is very strong, and the corresponding propene radical-cation at m/z=42 (colored orange), produced by elimination of propane, gives the base peak. By having the six carbons of hexane closed to a ring, the fragmentation is profoundly changed. To begin with, the molecular ion at m/z=84 is much stronger than the corresponding ions in the previous acyclic compounds. The base peak at m/z=56 is produced by loss of ethene, so it is an odd-electron ion (colored orange). The alkenyl cations at m/z=41 & 27 are stronger than the corresponding alkyl cations (m/z=43 & 29). The loss of methyl (m/z=69), and a corresponding small m/z=15 ion obviously require some hydrogen rearrangements. Fragmentation: aldehydes and ketones Pentanal displays a set of ions associated with the alkyl chain (e.g. m/z=57, 43, 41, 29 & 27). The molecular ion at m/z=86 is very weak, as is the M-1 ion at m/z=85 (aldehydes generally show fragment ions involving loss of groups attached to the carbonyl function). Thus, the m/z=29 ion is probably composed of both ethyl and HCO cations. Odd-electron rearrangement ions are observed at m/z=58 (possibly loss of CO) & 44 (rearrangement involving loss of propene). The molecular ion at m/z=86 is more abundant than in the previous aldehyde spectrum. The directive effect of the carbonyl group on fragmentation is also apparent here. Loss of carbonyl substituents by alpha-fragmentation include: loss of methyl (M-15 at m/z=71) and loss of a propyl radical (M-43 at m/z=43). These comprise the most prominent fragment ions. The molecular ion at m/z=86 is about as strong as in 2pentanone. Because of the symmetry, ethyl groups are the only carbonyl substituents that can be lost by an alphafragmentation. The strongest fragment ions are found at m/z=57 (loss of an ethyl group) and m/z=29 (an ethyl cation). Fragmentation: Alcohols and Sulfides In 1-pentanol the hydroxyl group is at the end of the fivecarbon chain. There are two significant odd-electron fragment ions, one at m/z=70 (loss of water), and the other at m/z=42 (loss of water and ethene). The fragment ion at m/z=55 is probably due to a methyl radical loss from the m/z=70 ion. The m/z=31 ion may be a protonated formaldehyde ion, formed by alpha-fragmentation. 3-Pentanol shows three significant fragment ions. Alphafragmentation (loss of an ethyl radical) forms the m/z=59 base peak. Loss of water from this gives a m/z=41 fragment, and loss of ethene from m/z=59 gives a m/z=31 fragment. The molecular ion (m/z=90) is strong, and the presence of sulfur is indicated by a larger than usual M+2 (m/z=92) peak. This is true for the m/z=75 & 47 peaks as well. Loss of methyl and ethyl radicals generate ions at m/z=75 & 61. The odd-electron rearrangement ion at m/z=62 results from loss of ethene. Finally, m/z=47 may be formed from m/z=62 by loss of a methyl radical. Fragmentation: Amines The mass spectrum of 1-aminopentane is remarkably simple, thanks to the directive influence of nitrogen. Alphafragmentation generates the m/z=30 even-electron cation, which is the only significant fragment ion. The molecular ion (m/z=87) is rather weak. The mass spectrum of 3-aminopentane is only slightly more complex than its isomer above. The molecular ion is very weak, and alpha-fragmentation again provides the base peak (m/z=58). Loss of ammonia from this ion generates the m/z=41 ion. The cyclic secondary amine, piperidine, has a more complex mass spectrum. The molecular ion at m/z=85 is relatively strong. Alpha-fragmentation of a hydrogen gives the M-1 base peak. Because of the ring, alpha-fragmentation of a carbon group does not result in a change of mass. This ring-opened cation-radical may then lose an ethyl radical or ethene to give respectively m/z=56 & 57 ions. Loss of an allyl radical from the ring-opened molecular ion would produce the m/z=44 ion. Fragmentation: Esters and amides The molecular ion in the mass spectrum of ethyl acetate is rather weak. The base peak results from an alphafragmentation of ethoxyl radical to give an m/z=43 ion. It is interesting that the alternative alpha-cleavage to an M-15 ion is very weak. The loss of water to generate the odd-electron ion at m/z=70 is curious. Small methyl and ethyl ions are found at m/z=15 & 29. The isomeric ester, methyl propanoate, has a more abundant molecular ion than ethyl acetate. The alpha fragmentation of methoxyl radical generates the strong m/z=57 ion. Alphafragmentation of the ethyl group leads to both m/z=59 & 29. A smaller methyl peak is also seen. The stability of dimethylformamide (DMF) is evident in the abundance of its molecular ion (m/z=73), which is also the base peak. A small M-15 peak is observed, but the second most abundant ion is loss of HCO by an alpha-cleavage (m/z=44). Other ions at m/z=42, 30 & 28 are probably derived from the m/z=44 ion. What are these? Isomer Differentiation 1,1,2,2-tetrafluoroethane. The nearest large fragment ion to the small molecular ion is at m/z=83, a loss of 19 amu. This suggests loss of fluorine. The m/z=51 ion represents half the molecule. A hydrogen shift is necessary to explain the m/z=33 ion. 1,1,1,2-tetrafluoroethane. Important differences from isomer 1 are that the m/z=51 ion is much smaller, m/z=33 is much larger, and a new strong ion at m/z=69 has appeared. Since fluorine is present, this may be assigned as a trifluoromethyl cation. Allylic Cleavage This is a major type of fragmentation for alkenes leading to alkyl radical loss. Unfortunately, migration of the double bond occurs before fragmentation so that the observation of ions of this type is of little structural value. The mass spectra of many alkenes, especially polyenes, tend to be independent of the position of the double bond so that isomers cannot be distinguished. The m/z 41 ion is the most common ion observed in the mass spectra of aliphatic compounds, together with homologues of m/z 55, 69, 83 . . . Allylic ions at m/z 41, 55, 69, 83 M+. Charge Site Initiation (Inductive Cleavage) Pentene elimination followed by alpha cleavage [HOCH2]+ [-C2H5]+ 3e rearrangement Pentene elimination followed by H• loss C3H7+ C2H5+ i i M+. [-CH3] α Pentene elimination [-C2H5] 3e rearrangement C3H7+ i [C2H3]+ α [-CH3] M+. i [-CH3] α Rearrangement Reactions McLafferty Rearrangement – 1 γ-H rearrangement McLafferty Rearrangement – 2 γ-H rearrangement Deuterium labelling studies show that a g-H atom is transferred quite specifically through a sixmembered transition state. If there are no g-H atoms, the rearrangement does not occur. Note that substituents on the a-carbon atom are retained in the ion but substituents on the b- and gcarbon atoms are lost as part of the alkene - useful in locating site of branching in an alkyl chain. [C2H5CO] + No alkyl chain 3 or more C atoms long so no McLafferty Rearrangement leading to alkene loss [C2H5] + M+. [C3H7]+, [CH3CO] + C2H4 loss from C3H7 alkyl chain CH3 loss M+. Loss of C4H8 from C5H11 alkyl chain [MC5H11]+ [M-OCH3]+ M+. Loss of C4H8 from C5H11 alkyl chain [M-NH2]+ M+. McLafferty Rearrangement – 3 γ-H rearrangement If an even mass fragment ion is found which could be formed from the molecular ion by the loss of 28, 42, 56, 70, . . . Da., always suspect that it results from a McLafferty rearrangement or of a related process. The driving force for the rearrangement is the formation of a strong bond between the H atom and the unsaturated heteroatom carrying the charge. Similar reactions occur with H-transfer to other heteroatoms. Self Assessment • In a long chain fatty acid, draw an inductive cleavage mechanism and an alpha cleavage mechanism. • Also draw the Mclafferty rearrangment for the long chain fatty acid. What masses would be expected? • A peak at m/z 31 suggests what moiety? 47? • List two ways that isomers can be differentiated in mass spectrometry. • Can stereoisomers be differentiated using MS?