BUILDING COVALENT MOLECULES

BUILDING COVALENT MOLECULES
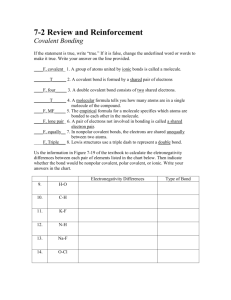
1.
In covalent molecules the electrons are ____________________ so that each atom can get a
__________ ____________________ ____________________.
2.
The sharing of electrons is always done in _______________ of electrons.
# Shared
Electrons
2
4
Name of Bond Symbol Model Representation
6
3.
We will be using the following elements when we build covalent compounds:
Element Lewis Dot Diagram
Covalent bonds needed to fill outer shell
Color
Hydrogen
Oxygen
Sulfur
Nitrogen
Carbon
Halogens (F/Cl)
4.
Suppose you need to make three covalent bonds to get a full outer shell. What are three ways of covalent bonding involving combinations of single, double, and triple bonds that you could use?
Use nitrogen as an example.
5.
When you build a good model what happens to the holes in the atomic models?
6.
If you have to use more than two atoms, how do you decide which element goes in the middle?
Molecule Name Chemical Formula
Water
(Dihydrogen
Monoxide)
H
2
O
Rotten Egg Gas
(Dihydrogen
Sulfide)
H
2
S
Lewis Structure
Ammonia NH
3
Methane CH
4
Fluorine F
2
Oxygen O
2
Nitrogen N
2
Hydrogen Cyanide HCN
Carbon Dioxide CO
2
Structure Drawing
(Draw what you see)
Molecule Name Chemical Formula
Methyl Chloride CH
3
Cl
Lewis Structure
Structure Drawing
(Draw what you see)
Methanol
CH
3
OH
(Hint: Build CH
3
, then OH, and then put them together.)
Propane
Butane
CH
3
CH
2
CH
3
(C
3
H
8
)
(Hint: Build CH
3
, CH
2
, CH
3
, and then put them together.)
CH
3
CH
2
CH
2
CH
3
(C
4
H
10
)
(Hint: See notes above.)
Pentane
CH
3
CH
2
CH
2
CH
2
CH
3
(C
5
H
12
)
(Hint: See notes above.)
Pentane can form different structures, not just one long chain. Using your pentane molecule, try rearranging the atoms (without changing the number of each atom) into different forms. Draw the different structures below. You should have two different structures from the original structure.
Pentane can also form a ring called cyclopentane (C
5
H
10
). From your pentane structure, remove two hydrogen atoms. Then rearrange the remaining atoms to form a ring. Draw the structure below.






![QUIZ 2: Week of 09.03.12 Name: [7pts] 1.) Thoughtful list of 3](http://s3.studylib.net/store/data/006619037_1-3340fd6e4f1f4575c6d8cf5f79f0ff3e-300x300.png)