Ionic Bonding
advertisement

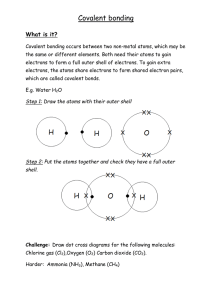
Name ________________ Ionic Bonding Ionic bonding occurs when a metal transfers one or more electrons to a non-metal in an effort to fill its outer shell (the so called “octet rule”). Atoms with a high electonegativity will become electron acceptors and atoms with a low electronegativity will be come electron donors. We can depict the formation of sodium chloride (table salt) as such. Notice that the result is two atoms held together by unlike charges (like magnets). Na Cl Na Cl If we are to show the formation of an ion like Calcium Chloride it works a little different. Calcium needs to get rid of two electrons to get to a full shell, and Chlorine can only take one. At this point we need to two chlorines to act as electron acceptors. In order to make full shells you can always add more atoms if your need to. Cl Ca Cl Ca Cl2 Show the transfer of electrons for the following ionic bonds. 1. K + F 2. Mg+ I 3. Be + S 4. Na + O 5. Al + Br 2 Name ________________ Covalent Bonding A covalent bond occurs between tow or more not metals trying to obtain full outer shells of electrons (stable octets). There is little difference in electronegativity between most non-metals. As a result, there is no transfer of electrons. Instead atoms are force to share electrons in order to get their full shells. H Cl H Cl Diagram the covalent bonds that result from the following groups of atoms. Notice sometimes you will be bonding together more than two atoms. Make sure to draw each atom first with its dot structure to show how many valence electrons it has. 6. F + F 7. O + O 8. N + N 9. C + O + O 10. H+H+O