ApalEngcongActivity1
advertisement

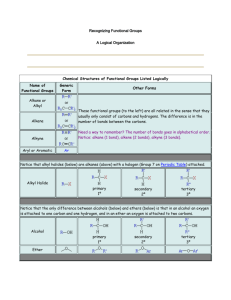
WHAT IS MORPHINE -- ACTIVITY #1 What is morphine? What is it used for? Morphine is a potent opiate analgesic psychoactive drug and is considered to be the prototypical opioid. In clinical medicine, morphine is regarded as the gold standard, or benchmark, of analgesics used to relieve severe or agonizing pain and suffering. Like other opioid, morphine acts directly on the central nervous system (CNS) to relieve pain. Morphine has a high potential for addiction; tolerance and psychological dependence develop rapidly, although physical addiction may take several months to develop. Morphine can be used as an analgesic to relieve pain in myocardial infarction, pain in sickle cell crisis, pain associated with surgical conditions, pain associated with trauma, severe chronic pain, pain from kidney stones and severe back pain. Morphine can also be used as an adjunct to general anesthesia, in epidural anesthesia or intrathecal analgesia, for palliative care, as an antitussive for severe cough, for treatment of dyspnea, as an antidiarrheal in chronic conditions, for remarkable relief of acute pulmonary edema through an unknown mechanism, to lower and stabilise blood glucose in diabetics and combat other diabetic effects including diabetic neuropathy. What is heroin? What do you know about it? Heroin, or diacetylmorphine, also known as diamorphine, is a semi-synthetic opioid drug synthesized from morphine, a derivative of the opium poppy. It is the 3,6-diacetyl ester of morphine. The white crystalline form is commonly the hydrochloride salt diacetylmorphine hydrochloride, though often adulterated thus dulling the sheen and consistency from that to a matte white powder, which heroin freebase typically is. 90% of Heroin is said to be produced in Afghanistan. As with other opioids, heroin is used as both an analgesic and a recreational drug, and has a high potential for abuse. Frequent and regular administration is associated with tolerance and physical dependence, which may develop into addiction. We know that heroin is a drug, which has a high potential for addiction. When taken in large quantity, it can greatly affect the Central Nervous System. Questions: What do they have in common? Morphine and heroine are both analgesic drugs and opioid drugs. Since heroine is synthesized from morphine, they contain some common components. Furthermore, Morphine and heroin are white, crystalline powders. The major pharmacological effects of heroin can be traced back to structural properties of the morphine molecule, and heroin shares a similar pharmacological profile with morphine: both induce analgesia, hypothermia, sedation, inhibition of intestinal motility, and depression of the immune function. What are the functional groups? The organic functional groups are the following: Alkane, Alkyl halide, Primary alcohol, Secondary alcohol, Tertiary alcohol, Aldehyde, Ketone, Carboxylic acid, Carbonyl function, Ester, Amide, Primary amine, Secondary amine, Tertiary amine, Acid chloride, Acid anhydride, Nitrile, Carboxylate ion or salt, Ammonium ion, Amino acid, Alkene, trans-Alkene, cis-Alkene, Ether, Alkoxide ion, Hydroxynitrile, Primary carbocation, Secondary carbocation, Tertiary carbocation, Acyl cation, Polymer, Diol or polyol. Among the many functional groups, morphine has tertiary amine, phenol, ether and allylic alcohol functional groups. Alkane - Alkyl, and occasionally aryl (aromatic) functions are represented by the R- Alkyl halide - Alkyl halides [haloalkanes] consist of an alkyl group attached to a halogen Primary alcohol - Primary alcohols have an -OH function attached to an R-CH2- group. Primary alcohols can be oxidised to aldehydes and on to carboxylic acids. (It can be difficult to stop the oxidation at the aldehyde stage.) Secondary alcohol - Secondary alcohols have an -OH function attached to a R2CH- group. Secondary alcohols can be oxidised to ketones. Tertiary alcohol - Tertiary alcohols have an -OH function attached to a R3C- group. Tertiary alcohols are resistant to oxidation with acidified potassium dichromate(VI), K. Aldehyde - Aldehydes have a hydrogen and an alkyl (or aromatic) group attached to a carbonyl function. Aldehydes can be shown in text as: RCHO. Aldehydes are easily oxidised to carboxylic acids, and they can be reduced to primary alcohols. Aldehydes can be distinguished from ketones by giving positive test results with Fehlings solution (brick red precipitate) or Tollens reagent (silver mirror). Aldehydes give red-orange precipitates with 2,4-dinitrophenyl hydrazine. Ketone - Ketones have a pair of alkyl or aromatic groups attached to a carbonyl function. Ketones can be shown in text as: RCOR. Ketones can be distinguished from aldehydes by giving negative test results with FehlingÕs solution (brick red precipitate) or Tollens reagent (silver mirror). Ketones give red-orange precipitates with 2,4-dinitrophenyl hydrazine. Carboxylic acid - Carboxylic acids have an alkyl or aromatic groups attached to a hydroxycarbonyl function. Carboxylic acids can be shown in text as: RCOOH. Carboxylic acids are weak Bronsted acids and they liberate CO2 from carbonates and hydrogen carbonates. Carbonyl function - The carbonyl group is a super function because many common functional groups are based on a carbonyl, including: aldehydes, ketones, carboxylic acids, esters, amides, acyl (acid) chlorides, acid anhydrides Ester - Esters have a pair of alkyl or aromatic groups attached to a carbonyl + linking oxygen function. Esters can be shown in text as: RCOOR or (occasionally) ROCOR. carboxylic acid + alcohol -> ester + water This is an acid catalysed equilibrium. Amide - Primary amides (shown) have an alkyl or aromatic group attached to an amino-carbonyl function. Primary amides can be shown in text as: RCONH2 Secondary amides have an alkyl or aryl group attached to the nitrogen: RCONHR Tertiary amides have two alkyl or aryl group attached to the nitrogen: RCONR2 Acid chloride - Acid chlorides, or acyl chlorides, have an alkyl (or aromatic) group attached to a carbonyl function plus a labile (easily displaced) chlorine. Acid chlorides highly reactive entities are highly susceptible to attack by nucleophiles. Acid anhydride - Acid anhydrides are formed when water is removed from a carboxylic acid, hence the name. Nitrile - Nitriles (or organo cyanides) have an alkyl (or aromatic) group attached to a carbontriple-bond-nitrogen function. Nitriles can be shown in text as: RCN. Note that there is a nomenclature issue with nitriles/cyanides. If a compound is named as the nitrile then the nitrile carbon is counted and included, but when the compound is named as the cyanide it is not. Carboxylate ion or salt - Carboxylate ions are the conjugate bases of carboxylic acids, ie. the deprotonated carboxylic acid. Ammonium ion - Ammonium ions have a total of four alkyl and/or hydrogen functions attached to a nitrogen atom Amino acid - Amino acids, strictly alpha-amino acids, have carboxylic acid, amino function and a hydrogen attached to a the same carbon atom. There are 20 naturally occurring amino acids. All except glycine (R = H) are chiral and only the L enantiomer is found in nature. Alkene - Alkenes consist of a C=C double bond function. Alkanes are planar as there is no rotation about the C=C bond. Alkenes are electron rich reactive centres and are susceptible to electrophilic addition. trans-Alkene - trans-alkenes are 1,2-disubstituted functions with the two R, X or other groups on opposite sides of the C=C function. Due to the non-rotation of the C=C bond, cis and trans geometric isomers are not [thermally] Interconverted. cis-Alkene - cis-Alkenes are 1,2-disubstituted functions with the two R, X or other groups on the same side of the C=C function. Due to the non-rotation of the C=C bond, cis and trans geometric isomers are not [thermally] Interconvertion. Ether - Ethers have a pair of alkyl or aromatic groups attached to a linking oxygen atom. Ethers are surprisingly unreactive and are very useful as solvents for many many (but not all) classes of reaction Alkoxide ion - Alkoxide ions an alkyl group attached to an oxyanion. Sodium alkoxides, RONa, are slightly stronger bases than water and so cannot be prepared in water. Instead they are prepared by adding sodium to the dry alcohol. Hydroxynitrile - Hydroxynitriles (also called cyanohydrins) are formed when hydrogen cyanide, H+ CN–, adds across the carbonyl function of an aldehyde or ketone. Primary carbocation - Primary carbocations have a single alkyl function attached to a carbon centre with a formal positive charge. Carbocations - also and more correctly called carbenium ions - are important reactive intermediates implicated in electrophilic addition reactions and electrophilic aromatic substitution reactions. Secondary carbocation - Secondary carbocations have a pair of alkyl functions attached to a carbon centre with a formal positive charge. Tertiary carbocation - Tertiary carbocations have three alkyl functions attached to a carbon centre with a formal positive charge Acyl cation - Acyl cations have an alkyl (or aromatic) group attached to a carbonyl function with a formal positive charge. Acyl cations are important reactive intermediates and are implicated in electrophilic addition reactions and electrophilic aromatic substitution reactions. Acyl cations are commonly formed from the corresponding acyl/acid chloride plus aluminium chloride. Polymer - Polymers consist of small monomer molecules that have reacted together so as to form a large covalently bonded structure. There are two general types of polymerisation: addition and condensation. Linear chain polymers are generally thermoplastic, while three dimensional network polymers are not. Diol or polyol - Diols and polyols are alcohols with two or more -OH functions. Diols and polyols are very soluble in water. They are used as high temperature polar solvents. Source: http://www.chemistry-drills.com/functional-groups.php?q=simple Use the following references: Morphine, see http://en.wikipedia.org/wiki/Morphine Heroin, see http://en.wikipedia.org/wiki/Heroin