Recognizing Functional Groups
advertisement
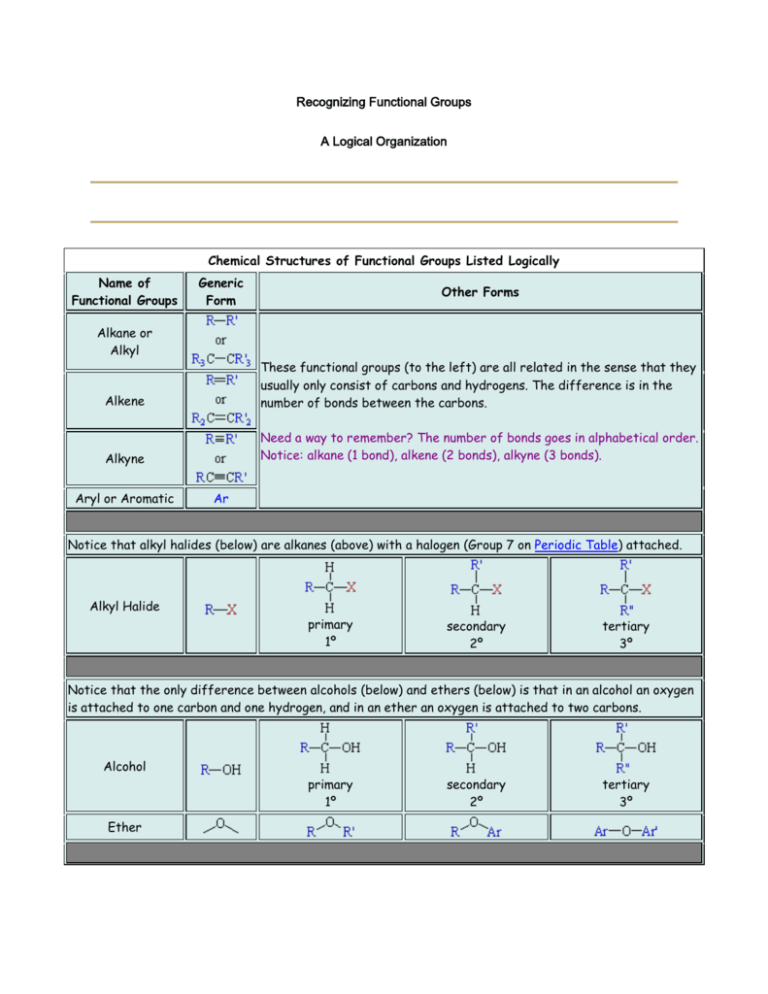
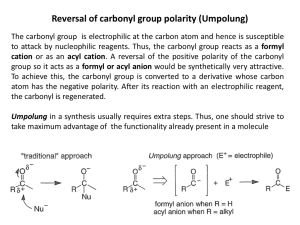
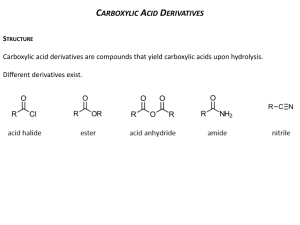
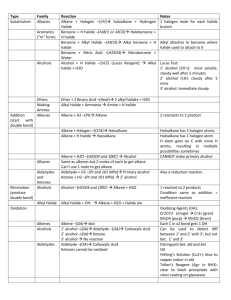
Recognizing Functional Groups A Logical Organization Chemical Structures of Functional Groups Listed Logically Name of Functional Groups Generic Form Alkane or Alkyl Other Forms Alkene These functional groups (to the left) are all related in the sense that they usually only consist of carbons and hydrogens. The difference is in the number of bonds between the carbons. Alkyne Need a way to remember? The number of bonds goes in alphabetical order. Notice: alkane (1 bond), alkene (2 bonds), alkyne (3 bonds). Aryl or Aromatic Ar Notice that alkyl halides (below) are alkanes (above) with a halogen (Group 7 on Periodic Table) attached. Alkyl Halide primary 1º secondary 2º tertiary 3º Notice that the only difference between alcohols (below) and ethers (below) is that in an alcohol an oxygen is attached to one carbon and one hydrogen, and in an ether an oxygen is attached to two carbons. Alcohol primary 1º Ether secondary 2º tertiary 3º Carbonyl Acyl Notice that a carbonyl/acyl is present in the next six functional groups! However, depending on what is attached to the these changes the name of the functional group! Notice the difference between a carbonyl and and acyl is that in the acyl we are including the attached carbon chain. Aldehydes have a carbon and a hydrogen attached to the carbonyl, or they have a hydrogen attached to the acyl. Notice that the oxygen must be on a terminal carbon. Aldehyde Ketone Need a way to remember? The name aldehyde comes from combining the words "dehydrogenated alcohol". So "al" from "alcohol" plus "dehyde" from "dehydrogenated" equals aldehyde. (Dehydrogenated means removing the hydrogen.) Ketones have two carbons attached to the carbonyl, or they have a carbon attached to the acyl. Notice that the oxygen cannot be on a terminal carbon. Now we come to the Carboxylic Acid group and all of its derivatives: Acyl/Acid Halides, Amides, and Esters. These groups also contain the carbonyl group, but here the acyl group is more often pointed out. Carboxyl or Carboxylic or Carboxylic Acid Acyl Halide or Acid Halide Carboxylic Acids have a carbon and an alcohol attached to the carbonyl, or they have an alcohol attached to the acyl. Carboxylic acids can only be on terminal carbons to maintain proper valence. Why two names? Acyl Halide comes from the fact that there is a halide attached to an acyl. Acid Halide comes from the fact that we have replaced the alcohol from a carboxyl group with a halide. You can also think of it as a carbon and a halide attached to a carbonyl! Amides have a carbon and a nitrogen attached to the carbonyl, or they have a nitrogen attached to the acyl. Amide Need a way to remember amides versus amines? Well, an amide is a carboxylic acid derivative. Both "amide" and "acid" have a "d" in them, "amine" does not. An Ester has a carbon on one side of the carbonyl and an oxygen attached to another carbon on the other side, or an oxygen is attached to both an acyl and to a carbon. Ester Need a way to remember? An ester is usually formed by reacting a carboxylic acid with an alcohol. So, the –OH on the carboxylic acid is replaced by the –OR from the alcohol. Amine primary 1º secondary 2º tertiary 3º Grignard or alkylmagnesium halide Nitrile Nitro Thiol Notice how Alkyl Halides, Alcohols, Amines can be primary, secondary, or tertiary. How do you tell? Well for Alkyl Halides and Alcohols, look at the carbon that the special group (–X, –OH) is attached to. How many carbons are attached to it? In a primary, only one is attached. In a secondary, two are attached. Finally in a tertiary, three carbons are attached. For Amines, look at how many carbons are attached to the nitrogen. In a primary, one carbon is attached and so on. What if there are no carbons attached? Then, it is an ammonia and not an amine at all!