1.3.3 Group VII Booklet(answers)
advertisement
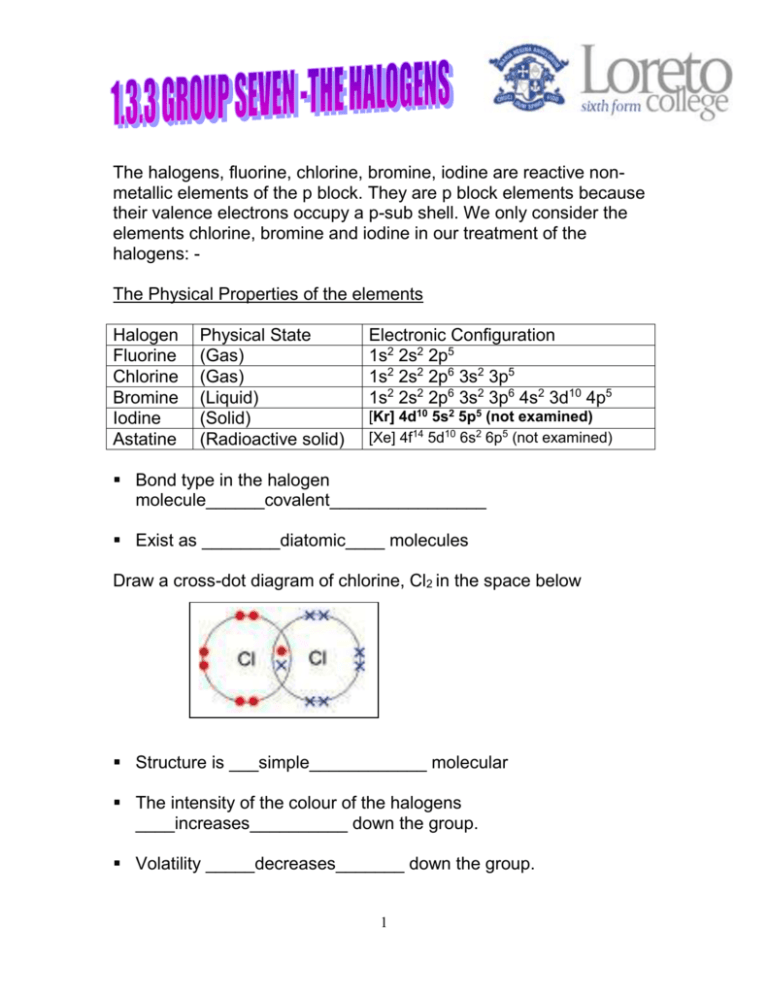
The halogens, fluorine, chlorine, bromine, iodine are reactive nonmetallic elements of the p block. They are p block elements because their valence electrons occupy a p-sub shell. We only consider the elements chlorine, bromine and iodine in our treatment of the halogens: The Physical Properties of the elements Halogen Fluorine Chlorine Bromine Iodine Astatine Physical State (Gas) (Gas) (Liquid) (Solid) (Radioactive solid) Electronic Configuration 1s2 2s2 2p5 1s2 2s2 2p6 3s2 3p5 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5 [Kr] 4d10 5s2 5p5 (not examined) [Xe] 4f14 5d10 6s2 6p5 (not examined) Bond type in the halogen molecule______covalent________________ Exist as ________diatomic____ molecules Draw a cross-dot diagram of chlorine, Cl2 in the space below Structure is ___simple____________ molecular The intensity of the colour of the halogens ____increases__________ down the group. Volatility _____decreases_______ down the group. 1 What do you understand by the term volatility? This term describes how easily a substance turns into a gas The group seven elements are described as being volatile and the elements become less volatile down the group. This means that the boiling points of the halogens increase down the group. (Obvious really as chlorine is a gas, bromine is a liquid and iodine is a solid). As we descend group seven the intermolecular forces between the molecules of the halogens increases. These intermolecular forces are known as _________Van der Waal’s_________________. The strength of these forces results from interactions of electrons between the molecules. The more electrons in the molecule the greater the induced diploes and the greater the attraction between the molecules. These increased attractions cause the boiling points to increase as these forces need to be broken. Chlorine Bromine Iodine Boiling point increases as the van der Waals forces increases due to the molecules containing more electrons. The van der waals forces become more difficult to break 2 Reactivity of the halogens The halogens all have seven electrons in their outer shells. A halogen atom readily accepts an electron to form a halide ion with a full shell of electrons (very stable) Reacting as oxidising agents All of the halogens act as oxidising agents. The most powerful is ____chlorine________. State and explain the trend in oxidising power down the group ………Decreases Less readily gains an electron due to decreased effective attraction of the nucleus for the electrons. Distance from nucleus of outer electrons is greater because number of shells and shielding is increasing down the group …………………………………………………………………………….. …………………………………………………………………………….. …………………………………………………………………………….. State and explain the trend in reactivity down the group Decreases …………………………………………………………………………….. Atomic radius increases Electron shielding increases The ability to gain an e into the p sub shell dereases ( to form a halide ion Effective nuclear attraction is weaker …………………………………………………………………………….. …………………………………………………………………………….. 3 You must be able to state and explain trends down group VII – look at the Group II booklet for similarities and differences. Don’t forget about atomic radii – this applies to group II and group VII. The decrease in reactivity of the halogens as the group is descended can be demonstrated by displacement reactions of the aqueous halides using chlorine, bromine and iodine. When chlorine is added (bubbled through) a solution of potassium iodide, the solution becomes brown due to the formation of iodine. If bromine is added to another solution of potassium iodide the same reaction would be seen. In both of these reactions the iodide ion is being oxidized to iodine I2(aq) + 2e- 2I-(aq) A fully balanced equation can be written for both of these reactions Br2 (g) + 2I-(aq) 2Br-(aq) + I2(aq) ** Cl2 (g) + 2I-(aq) 2Cl-(aq) + I2(aq) ** In both the above reactions iodide has been oxidized. This occurs because bromine and chlorine are stronger oxidizing agents than iodine. Iodine being the weakest oxidizing agent could not displace any of the others from their solutions. When chlorine is added to a solution of potassium bromide, the solution becomes yellow orange as bromine is released. This reaction shows that chlorine is a better oxidizing agent than bromine. This can be seen in the equation below Cl2 (g) + 2Br-(aq) 2Cl-(aq) 4 + Br2(aq) Be careful with terminology – nothing reacts with chloride. Watch for the traps in the questions! **Iodine in a non polar solvent is purple – watch out! 5 The halide ion can be determined in aqueous solution by the use of a solution of silver nitrate followed by the addition of dilute ammonia solution. When a solution of silver nitrate is added to a halide ion in solution the following observations are recorded. After the addition of the silver nitrate, ammonia solution is added to any of the precipitates formed. HALIDE ION OBSERVATIONS WITH SILVER NITRATE OBSERVATIONS WITH AMMONIA Soluble ( dilute ammonia) Cl- White ppt - Cream ppt Soluble( conc ammonia) Br Yellow ppt I Insoluble - Equations Write equations for the reactions between the halide ions and silver ions Cl- (aq)+ Ag+(aq) → AgCl(s) Br- (aq)+ Ag+(aq) → AgBr(s) I- (aq)+ Ag+(aq) → AgI(s) ……………………………………………………………….. …………………………………………………………………………….. …………………………………………………………………………….. 6 Reactions of the halogens with sodium hydroxide Chlorine, bromine and iodine disproportionate in alkali. Write an equation here for the reaction between NaOH and chlorine: 2NaOH + Cl2 →NaCl + NaOCl + H2O Also in hot conc alkali This is on Jan 10 paper Q4d For example in cold alkali (15OC) chlorine disproportionates to give chloride and chlorate ions. Chlorate is the ion present in bleach. In the equation the Oxidation State of the chlorine is given below the species in the balanced equation. Cl2 (g) + 2OH- (g) (0) Cl - (aq) + ClO-(aq) + H2O(l) (-1) (+1) Chlorine in water is both an acid and a bleach - watch your teacher demonstrate the action of chlorine water on indicator paper – there are two changes________________________________________ Chlorine reacts with water. This reaction is used in water purification. A disproportionation reaction occurs and hydrochloric acid and chloric(I) acid is formed Cl2 (g) + H2O (l) HCl(l) + HOCl (l) The bacteria in water are destroyed by reactive oxygen atoms which are produced by a slow decomposition reaction of the chloric acid = sterilisation HClO HCl + O 7 Some people think we should not put chlorine in drinking water – why? Chlorine is toxic in excess and it can react with hydrocarbons to form chlorinated hydrocarbons which are carcinogens. 8



![[1] - Boswellsgmt](http://s3.studylib.net/store/data/006603407_1-fadfbce8d94050a9fb3c38a07d86e8ee-300x300.png)