Expt 4. Stereochemistry - Winona State University
advertisement

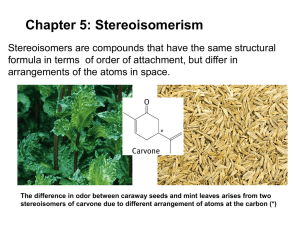
CHEM 350 Principles of Organic Chemistry I Lab Prof. T. Nalli, WSU Experiment 4 - Stereoisomerism: Models and Experiments. Relevant Reading – Smith, Chapter 5 Part A. Models OVERVIEW This part of experiment 4 introduces the concepts of chirality, enantiomerism, and diastereomerism through the construction of models of molecules that contain stereogenic centers. DEFINITIONS Stereogenic Center. A tetrahedral atom that has four different groups bonded to it is called a tetrahedral stereogenic center. For convenience throughout this document we refer to it simply as a stereogenic center. (Other older names to be discouraged are, "chiral center" and "asymmetric atom".) Plane of Symmetry. Any object that consists of two halves that are mirror images of each other is said to possess a plane of symmetry. For example a human body, a fork, a baseball bat, and a basketball all possess mirror planes (neglecting small imperfections and markings on the objects). Examples of objects that do not have a plane of symmetry would be a human hand, a golf club, and a bowling ball. Chiral. Chiral objects possess a "handedness". In other words, they exist as two different mirror-image forms, a "right-handed" and a "left-handed" form. Any object that is not identical to its mirror image is chiral. Achiral. Achiral objects do not possess a "handedness". They do not exist as two different mirror-image forms. Any object that is identical to its mirror image is achiral. Superimposable. This is the criterion we use to decide if molecules/models are identical to each other. If by turning a model around in space and/or rotating single bonds you can make it into a form that will superimpose atom for atom on a second model, that means the two models are identical. Superimposable models are identical. Stereoisomers. Stereoisomer is the word that describes isomers in which all of the atoms are attached in the same sequence. The only difference is the 3-D arrangement of the atoms. Examples of stereoisomers are cis/trans isomers, enantiomers, and diastereomers. Enantiomers. The two mirror-image forms of a chiral molecule are called enantiomers. Enantiomers are isomers that are mirror images of each other. Please note, in order to be isomers the structures must be non-identical. Hence, enantiomers are often defined as non-superimposable mirror images. Diastereomers. Stereoisomers that are not mirror images of each other. PROCEDURE Answer all questions directly in your lab notebook as observations. Draw wedge/dash representations of each molecule and/or pair of molecules as you study them. STEREOGENIC CENTERS AND SYMMETRY Make a model in which a tetrahedral carbon atom has four different color balls bonded to it. Observe it carefully. Does the model possess any planes of symmetry? This model can be used to represent any molecule that contains one stereogenic center. As an example consider 3-methylhexane. Draw the structure of 3-methylhexane in your notebook and use an asterisk to label the stereogenic center and name the four different groups attached to it. Note that the model with four different color balls can be used to represent 3-methylhexane by simply stipulating that each ball represents one of these four groups. Does 3-methylhexane possess any planes of symmetry? For further practice in finding tetrahedral stereogenic centers, draw structures of each of the following and mark all stereogenic centers present with an asterisk: 1-butanol, 2butanol, 1,2-butanediol, 1,3-butanediol, 1,4-butanediol, 2,3-butanediol. CHIRALITY AND ENANTIOMERS As you have just seen, the presence of one stereogenic center in a molecule causes it to not have a plane of symmetry. Objects that lack a plane of symmetry are usually chiral. (The only exceptions are when they possess other symmetry elements such as a center or axis of symmetry.) Which of the following molecules are chiral: 1-chlorohexane, 2-chlorohexane, 3chlorohexane, 1-chloropentane, 2-chloropentane, 3-chloropentane? Go back to your original model (4 different colors) and make a second identical model of it. Make sure the two models are identical by trying to superimpose them; all of the atoms on one should superimpose on atoms of the same color in the other. Now switch any two balls on one of the models. Are the models still superimposable? Are they identical? What is the word for non-identical structures that have the same molecular formula? Place the two models side-by-side on the desktop so that they both have the same color ball pointing up. Keep that atom pointing up and rotate the models so that one of the other colors is closest to the ball of the same color on the other model. (Like the picture on next page.) You should now be able to observe that the models are mirror images of each other. At the same time, they are clearly not identical. Hence, the two models represent enantiomers of each other. To resummarize all of your previous work, all molecules that possess one stereogenic center and, therefore, lack a plane of symmetry are chiral and exist as a pair of enantiomers. Again, switch any two balls on one of the models. Are the models still mirror images of each other? Does either possess a plane of symmetry? Are the models superimposable? Do the models represent identical or different structures? Do the models represent enantiomers? Why or why not? Note that switching any two groups on a stereogenic center creates the enantiomer of the molecule you started with. Another way of saying this is that it inverts the configuration of the stereogenic center. Make models of the two enantiomers of 2-chlorobutane in Chem3D. You should be able to build one of the enantiomers easily using procedures learned in Expts 1 and 3. (You can either draw the molecule using ChemDraw then copy and paste it into Chem3D or use the single bond tool in Chem3D to connect four carbons and then attach a Cl to the second carbon using the single bond tool and the atom “A” tool). To get its enantiomer, merely select the whole molecule (<ctrl>-A), then copy it (<ctrl>-C), open a new window (<ctrl>-N), paste it (<ctrl>-V), and then invert it (<ctrl>-I). Use molecular mechanics to calculate minimized energies for each of the enantiomers. What can be concluded about the energies of enantiomers based on this calculation? ACHIRAL MOLECULES Now replace the red ball on each of the models with a second white ball so that each now could be representing a CH2 group with two different things attached to it. Are the models mirror images of each other? Does either possess a plane of symmetry? Are the models superimposable? Do the models represent identical or different structures? Do the models represent enantiomers? Why or why not? Now switch any two balls on one of the models. Does this change anything in terms of the answers to the questions in the preceding paragraph? Objects that possess a plane of symmetry are never chiral. Explain why. When a tetrahedral atom has two identical groups attached there will almost always be a plane of symmetry cutting between the two identical groups. (The only exception would be if one of the two non-identical groups contained a stereogenic center or was in some other way chiral.) Molecules represented by the models used in this part of the lab are, therefore, not capable of existing as enantiomers and are said to be achiral. In other words, achiral molecules have mirror images that are identical to themselves. Which of the following objects are chiral: a baseball hat, a baseball glove, a baseball bat, a baseball, a baseball pitcher, a baseball diamond, a screw, a nail, a spiral staircase, the letter N, the letter T? MOLECULES WITH TWO STEREOGENIC CENTERS Two Stereogenic Centers with Identical Groups Remake the first model of the lab (4 different colors around a central carbon atom) and again make a second identical model of it. Make sure the two models are identical by superimposing them. Now remove the red ball from each and connect the two carbons to each other. How many stereogenic centers does this molecule possess? Does the molecule represented by the model possess a plane of symmetry in any of its conformations? Construct a new model that is the mirror image of the previously examined one. Are the two models identical or different? What term should be used to describe the relationship between the two models? Is this molecule chiral or achiral? Take one of the previous models and interchange any two groups on one of the carbons. Do the same with the other model. Do these models have a conformation that possesses a plane of symmetry? If so, draw that conformation (use a wedge/dash formula) and indicate the location of the mirror plane. Are the two models identical or different? Are the models mirror images of each other? Do the models represent stereoisomers of each other? Do the models represent enantiomers of each other? Is this molecule chiral or achiral? The two models just examined represent an example of a meso compound. The simplest meso compounds are compounds that contain two stereogenic centers but are still achiral because the two stereogenic centers are mirror images of each other leading to a plane of symmetry being present. A meso compound is only possible if the two stereogenic centers have the same four groups attached. Which of these compounds can exist as an achiral meso form; 2,3-dichlorobutane, 1,2dichlorobutane, 2,3-dichloropentane, 2,4-dichloropentane? The four models just looked at prove that a compound with two stereogenic centers that have identical groups attached can exist as three stereoisomers; a pair of enantiomers and a meso compound. Think about what the relationship is between either of the enantiomers (one of the first two models in this part – please feel free to remake one) and the meso form. Are they enantiomers of each other? Are they identical to each other? What is the relationship? (See definitions.) Use Chem3D molecular mechanics to calculate the relative energies of all three stereoisomers of 3,4-dimethylhexane. You can get to all three stereoisomers by first drawing the molecule in ChemDraw and copying and pasting into Chem3D. This will give you the meso isomer. Convert this model to one of the enantiomeric pair by switching the CH3 and H on one of the stereogenic carbons. You can then get the third stereoisomer by selecting the entire molecule and then inverting it as you did earlier in the lab. Two Stereogenic Centers with Different Groups Now replace one of the balls on each of the models with a different color ball. You should now have models of a compound with two stereogenic centers that do not have all four of the attached groups the same as each other. Does either of the models possess a plane of symmetry in any of its conformations? Are the two models identical or different? If different, state the relationship between them. How many stereoisomers exist for a compound such as this with two stereogenic centers that do not have the same groups attached? Explain and check your answer with the instructor. REPRESENTING MOLECULES WITH STEREOGENIC CENTERS The great German chemist, Emil Fischer, often worked with compounds with multiple stereogenic centers. In order to make representing these structures on paper easier, he devised the structural convention known as a Fischer projection. Go back to a simple model with a central carbon atom and four different colors attached. Hold the model so that two of the bonds are parallel to the floor and pointing straight at you. The other two bonds should be pointing away from you and be perpendicular to the floor. Verify that the molecule in this orientation is represented by wedge/dash representation shown at left below. C wedge/dash formula = Fischer projection A Fischer projection (or "cross formula") uses a simple cross to represent this orientation of a stereogenic center. Make models of each pair of molecules and decide if they are enantiomers or identical structures. CH3 H CH3 Br Br H OH H OH CH3 H CH3 Br H CH3 OH Br H CH3 OH OH CH3 OH H Br Br Br H OH OH Br CH3 Note that all of the above examples involve switching two groups on a Fischer projection. When the only difference between two Fischer projections is that two groups have been switched then the relationship between those two structures is what? Make models of each pair of molecules below and decide if they are enantiomers or identical structures. CH3 H H Br HO OH Br CH3 H Br OH Br OH Br OH H CH3 CH3 H CH3 CH3 Br OH H Use the above three examples to answer the following questions. When the only difference between two Fischer projections is that the projection appears to have rotated 90 then the relationship between the structures represented is what? When the only difference between two Fischer projections is that the projection appears to have rotated 180 then the relationship between the structures represented is what? When the only difference between two Fischer projections is that three of the attached groups have rotated then the relationship between the structures is? NAMING ENANTIOMERIC STRUCTURES; THE R AND S CONVENTION A way to refer to the specific configuration around a tetrahedral stereogenic center is obviously needed. In other words, one needs to be able to specify which enantiomer is being referred to without drawing it. The convention used by organic chemists is as follows: (1) The four groups attached to the stereogenic center are assigned priorities using the Cahn-Ingold-Prelog system (the same system used for assigning priorities in the E/Z naming of alkenes) (2) The molecule is oriented with the lowest priority group pointing away from you (3) The sense of rotation in going from the first priority group to the second to the third is noted - if the sense is clockwise then the stereogenic center is designated as "R", if the sense is counterclockwise then the stereogenic center is designated as "S" Make models if necessary to determine the R/S designation of all stereogenic centers in each of the following. CH2NHCH3 CH3 CH3 CH3 CH3 Br OH D H H Br CD3 OH N N OH N O H N O HO N H Crixivan Part B. Comparison of Enantiomers: (+)-Carvone and (-)-Carvone References: Pavia - Expt 15. Techniques 23-24. Overview - The physical and biological properties of the enantiomers, (+)- carvone and (-)-carvone, will be compared. Physical properties to be measured are the refractive index, the specific rotation, and the C-13 spectrum. The odors of the two carvones will also be assessed. Safety Precautions - CDCl3 has harmful fumes. Avoid breathing it and dispense it in a fume hood. Procedures: NMR. Selected teams will be asked to obtain an NMR spectrum of one of the carvone isomers. See the handout for experiment #2 and your lab notebook for the procedures used for sample preparation. The instructor will assist in obtaining the C-13/DEPT spectrum. The class results will be posted for all teams to use in their lab reports. Specific rotation. The polarimeter you will use is different from the one described in Pavia. It does use a sodium lamp as the light source. With the sample tube inserted you should rotate the eyepiece to achieve maximum darkness in the center of the field of vision. Negative rotations on this polarimeter are calculated by subtracting the reading from 360 degrees. First measure the rotation of a blank sample (water). Do three independent measurements. If the average is significantly off from 0º then you are doing something wrong. Then measure the carvone samples the same way. Note the temperature in the lab in the vicinity of the polarimeter - we will assume the temperature of the samples is the same as the room. Use a ruler to carefully measure the height of each liquid in the polarimeter tube. Refractive index. The procedure is similar to that described in Pavia on pp 829-830. (1) Turn the refractometer on using the switch at front bottom right of the instrument. (2) Make sure the mode selector is set to D. (3) Open the prism assembly cover. If the measuring prism surface is not clean then it should be cleaned with alcohol and then distilled water (see step 12). Any residue left on the prism will affect the accuracy of readings. (4) Apply 4-5 drops of the sample solution to the measuring prism surface using a pipet. Important: the face of the prism must be completely covered with liquid. Close the prism cover and position the illumination arm so that the exposed face of the upper prism is fully illuminated. (5) Turn the dispersion correction wheel (see diagram) so that the crosshair adjustment access hole is at the six o'clock position. (6) Rotate the coarse adjustment control (large knob on right hand side) to position the shadow line at the bottom of the field of view. (7) Rotate the eyepiece to bring the crosshair into focus (if it isn't already). (8) Move the shadow line to the crosshair reticle with the coarse adjustment control. (9) Rotate the dispersion correction wheel to eliminate any red or green color at the edge of the shadow line. (10) Turn the adjustment control to center the shadow line to the crosshair. The shadow line must be perfectly centered to obtain an accurate reading (Figure 3a). (11) Depress the READ button. The value of the test sample will be digitally indicated in the display window. Depressing the TEMP button will activate a temperature-sensing device located in the measuring prism. The display will digitally indicate the actual temperature of the measuring prism and sample. (Always record the temperature as well as the D reading..) (12) Open the prism assembly cover and clean the measuring prism surface with alcohol and then distilled water. Wipe with a clean soft cloth or lens tissue, but do not wipe the measuring prism surface while it is dry. Odor assessment. Early in the lab (before your sense of smell becomes overwhelmed) you should try to carefully detect and describe the odor of each carvone. Compare the odor to other substances familiar to you but qualify the comparison if necessary. Do not stick your nose in the bottles and sniff! Not only is this dangerous, it will be counterproductive as your olfactory sense is keenest when detecting lower levels of compound. Report: Part A. Models The results table should present the molecular mechanics results for 2-chlorobutane and 3,4-dimethylhexane. The answers to some of the key questions posed throughout the lab handout should be reformulated as conclusions. Use structural drawings (wedge/dash and or Fischer cross formulas) as necessary to illustrate. Part B. Present the refractive index, optical rotation and odor assessment results in one table. Make sure to use standard abbreviations (as shown in the reading assignments) for refractive index and optical rotation rather than your own (i.e., do not abbreviate refractive index as R.I.) Use the equation given in Pavia under Technique 18 to correct the D readings to the standard temperature of 20ºC. Also make sure you convert the observed optical rotations to specific rotations before reporting. (The equation is given in Pavia in Technique 17 as well as in Smith. ) (The carvones examined in lab were pure substances so use the literature density of each as the concentration term.) Include literature values for the refractive index and specific rotation and include the % error of each measurement in the table as well. Make a separate table of NMR results that lists the data for both carvones, assigns each peak to a particular carbon, and gives literature values of the chemical shifts. The table should make comparing the spectra of the two carvones easy. Questions/Discussion Points 1. Discuss the accuracy and precision of the refractive index and optical rotation measurements. Discuss sources of error in each. 2. What did this experiment demonstrate about the properties of enantiomers? Discuss at some length please.