Transfusion Related Acute Lung Injury
advertisement

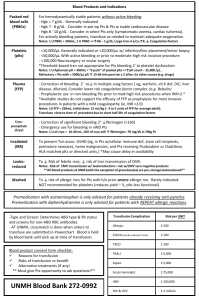
Transfusion Related Acute Lung Injury Introduction Although blood transfusions have become safer over time with expanded screening for infectious agents and leukocyte reduced blood products, risks still exist. Transfusion related acute lung injury (TRALI) is one of those risks and is now the most common cause of transfusion-related death in the United States 7. Because of its similarity to acute respiratory distress syndrome (ARDS), TRALI can be easily overlooked by clinicians in the critical care setting unless it is kept in their list of differentials for respiratory problems. Epidemiology TRALI has been reported to occur at a rate of 1:5000 units of blood transfused (0.02%/U over all blood products and 0.16% per patient transfused), although the actual rate may be higher if there are a significant number of unrecognized and unreported cases (1). Epidemiological studies of TRALI have been hindered both because its definition has changed over time as well as its recognition by clinicians. Studies have also been focused on different blood products at different times because of changing ideas about the pathophysiology of the disease. Literature searches have shown reports of TRALI with most all blood products. The table below was taken from Shander et al. and shows just how widely the incidence of TRALI seems to vary both between the blood products examined and the examiner reporting the incidence 1. 1 The true incidence of TRALI will remain fluid and elusive until a standardized definition of TRALI is developed and studies for the different blood products are redone under a single definition 4. Pathophysiology A Problem of Definition Although respiratory conditions in post-transfusion patients that resemble TRALI have been described since the 1950’s, “TRALI” as we know it and the attempt to define it did not begin until the 1980’s 7. Before the term TRALI was coined the same condition had also been called “pulmonary hypersensitivity reaction”, “allergic pulmonary edema”, “non-cardiogenic pulmonary edema”, and “pulmonary leucoagglutinin reaction” 7. The National Heart, Lung and Blood Institute originally defined TRALI as “new acute lung injury (ALI) occurring during or within 6 hours after a transfusion, with a clear temporal relationship to transfusion, in patients without or with risk factors for ALI other than transfusion.” 2. This definition was subsequently modified by a Canadian consensus conference in April of 2004 to be more clinically useful 2. The creation of a “Possible TRALI” diagnosis category, although its utility may be debated, was designed to simultaneously minimize the underreporting of TRALI and 2 yet recognize the complex clinical background of possible causes of ALI against which these patients are experiencing symptoms 2. A Problem of Pathology Our understanding of the pathogenesis of TRALI is evolving and incomplete. There are two schools of thought about the mechanism of TRALI: 1. TRALI may be caused by the interaction of donor leukocyte antibodies with recipient neutrophils 2. This results in neutrophil activation and aggregation in pulmonary capillaries and increased capillary leak 2. 2. TRALI may occur by a “two-hit” mechanism in which a neutrophil activation and aggregation can occur without leukocyte antibodies. Trauma and sepsis may induce pulmonary endothelial activation, thereby priming neutrophils to be activated by a variety of chemicals in blood products such as lipids, cytokines or antibodies 2. Again, this results in neutrophil activation and aggregation in pulmonary capillaries and increased capillary leak 2. Immune (Antibody)-Mediated Mechanism of TRALI The antibody theory maintains that TRALI is secondary to antibodies in donor plasma that are cogent for antigens found on recipient leukocytes 7. These antibodies activate complement and bind circulating neutrophils 7. This causes neutrophil sequestration within the vascular endothelium, and their subsequent activation 7. This neutrophil activation causes endothelial damage that in turns causes capillary leak and exudative pulmonary edema 7. The role of neutrophils causing lung damage in TRALI has been supported by pathological studies of patient who have died from TRALI which have shown immunoreactive neutrophils lining the alveolar capillaries 2. Leukocyte antibodies with associated antigen on recipient neutrophils have been reported in 65-90% of reported clinical cases, although there is debate as to whether this number is subject to reporting bias due to researchers not reporting cases as TRALI when there is no relevant antibody match detected 2. Antibodies that have been implicated in TRALI include 2: 1. HLA class I 2. HLA class II (directed at monocytes or pulmonary endothelium, as neutrophils do not have class II antigen) 3. Neutrophil-specific antibodies (most commonly directed at the HNA-3a antigen) 4. Preformed recipient leuko-agglutinating antibody (directed at transfused donor neutrophils) The fourth of the above list may account for a minority of cases (around 10%) of TRALI and be more significant in regards to granulocyte transfusions 6. Clinical studies on donor plasma implicated in TRALI reactions have demonstrated anti-HLA and antigranulocytic antibodies, but never with a 100% relationship 7. Retrospective analysis of donor plasma after TRALI reactions has shown that single donors with antibodies could cause TRALI in multiple patients, but often times did not 7. These opened the door to the idea that antibodies alone were insufficient to cause TRALI 7. 3 Two-Hit Mechanism of TRALI There are many perceived shortcomings in the antibody hypothesis. One such shortcoming was the fact that 15% of cases demonstrated no evidence of an antibodymediated mechanism 2. This 15% number may be lower though if more sensitive assays for HLA and neutrophil antibodies are used or assays for antibodies to other cell types are performed 2. Also, cases of TRALI do not always occur even when known antibodies are transfused into patients with the appropriate antigen 2. There is also a poor correlation between the frequency of TRALI and the amount of plasma in the transfused product 1. This seems to contradict the antibody hypothesis, which would predict increasing amounts of antibody with increasing amount of plasma 1. 1. 2. 3. 4. 5. Limitations of the Antibody Mediated Theory of TRALI In 15% of TRALI cases no related donor antibody can be found Even if an antibody can be implicated in a case of TRALI, a corresponding recipient antigen can not always be found Although HLA antibodies are commonly found in female donors, only a small proportion of these donors are implicated Known HLA antibodies have been transfused into recipients with corresponding antigen with no following TRALI reaction Incidence of TRALI not correlated with amount of plasma in products The two-hit hypothesis was born out of attempts to explain these contradictions 1. The first hit is related to the condition of the recipient and the second hit related to factors found in the donor blood 1. Hit #1 - Recipient Factors Silliman and colleagues first proposed the two-hit hypothesis after performing a comparison of 10 patients with TRALI to a control group of 10 patients who had febrile or urticarial transfusion reactions 1. They found that all of the 10 in the TRALI group had what they deemed significant clinical factors (such as infection, recent surgery, or cytokine administration) as opposed to only 20% of the control group 1. Also, they found a lack of significant titers of donor antibodies in the TRALI group 1.This lead them to propose that recipient factors played an important role in the development of TRALI. The recipient factor may be any event that primes polymorphonuclear lymphocyte activity 1. This priming activity is considered necessary but not sufficient criteria for the induction of TRALI. The primed neutrophil becomes comparable to a bullet loaded into a gun. The primed neutrophils are then preferentially sequestered in the pulmonary endothelium 5. The pulmonary vascular bed is unique in that PMNs are larger than many of the capillaries 6. PMNs must therefore change shape to pass through them 6. This slows them and allows for greater interaction time between the PMNs and endothelium and therefore greater time for interaction with receptors on the endothelium and adhesion to the endothelium 6. Hit #2 - Donor Factors The second hit is any substance found in the donor blood product that reacts with and activates the primed neutrophils. The second hit may be classic TRALI antibodies 4 (such as the anti-HLA, or antigranulocytic antibodies discussed in the immune-mediated mechanism) or “bioactive lipids” and cytokines in stored blood products 5. Although, antibodies can be implicated in the two-hit theory, it is the non-antibody substances that are most often discussed and researched 8. And since antibodies have been previously discussed in this paper we will also focus on the latter class of non-antibody molecules at this time. This class of molecules consists of cytokines and lipid-based substance that together are called biological response modifiers (BRMs) 8. BRMs have the property of stimulating the adherence and activation of neutrophils 8. An example of a cytokine that has been implicated as a BRM is CD40 7. BRM bioactive lipids consist of a mixture lysophosphatidylcholines that have been shown to prime the PMN oxidative burst or activate primed adherent PMNs in vitro 6. A common characteristic of these BRMs is that they concentrate in the cellular transfusion products over time 8. In one prospective case-control study of 81 TRALI reactions, TRALI was associated with increasing age of the transfused platelets 7. Not only does the concentration of these inflammatory cytokines and lipids increase with storage time, but they also decrease with pre-storage leukocyte reduction 7. Despite this, attempts to control TRALI by manipulation of storage time and leukocyte reduction have not been shown to consistently decrease the incidence of TRALI 7. The Problem of Two Theories The two theories regarding the mechanism of TRALI are not mutually exclusive. Both appear possible and so both may have an element of truth, although neither may tell the complete story. Antibodies may be the inciting factor in either theory and the antibody theory doesn’t exclude the existence of BRMs. The main problem in having two different theories lays not so much in Occam’s razor as in the difficulty of applying these theories to the prevention of TRALI. These two theories predict that TRALI will occur in different clinical circumstances. In the antibody theory, blood products with the highest antibody component such as FFP should have the highest incidence of TRALI 8. Whereas in the BRM based model of the two-hit hypothesis, blood products with cellular components are needed to cause TRALI 8. The backward prediction of the pathogenesis of TRALI reactions from epidemiological studies is complicated by all the problems discussed in the epidemiology section of this paper (a changing definition and focus on studying particular blood products that the fit the assumptions of the authors). Which pathogenic theory one accepts may drive which actions one thinks is necessary to prevent TRALI 8. This will be discussed further in the section on prevention. Clinical Presentation The key to the successful identification of a TRALI reaction in a patient may be the time frame of between the appearance of symptoms and the transfusion 1. Symptoms usually occur within 1-2 of the transfusion and will always appear within 6 hours 1. A major problem with the diagnosis of TRALI is that its clinical presentation mirrors that of ARDS 2. Symptoms exhibited by patients include: 1. Dyspnea/respiratory distress requiring oxygen support or mechanical ventilation 5 2. Hypoxemia and possibly cyanosis 3. Hypotension 4. Fever The onset of these symptoms is often abrupt 4. Chest imaging of TRALI patients shows pulmonary edema and bilateral pulmonary infiltrates 7. There are however no laboratory tests that can be ordered to confirm the diagnosis of TRALI 7. Diagnosis No consensus algorithm is available yet but there are recommendations for TRALI diagnosis 1. A working differential diagnosis would include 4: 1. Underlying pulmonary disease 2. Underlying cardiac disease 3. Transfusion-associated circulatory overload (TACO) 4. Severe allergic or anaphylactic reactions 5. All the risk factors for ALI In TRALI there is no evidence of circulatory overload, an absence of jugular venous distention, an absence of an S3 gallop, a normal central venous pressure and a normal pulmonary wedge pressure 2. This is important in distinguishing TRALI from the very similar condition of TACO. Also in TACO there is a posttransfusion-to-pretransfusion BNP ratio of greater than 1.5 2. A history of heart disease or positive fluid balance should also help establish the pretest probability of TACO vs. TRALI 3. Laboratory findings are of limited utility because they are not diagnostic of TRALI 2. Immunologic test of donor blood for HLA or neutrophil-specific antibodies and for the corresponding receptors on recipient neutrophils take days to weeks and so is also of limited utility for diagnosing TRALI 2. Although there are no set guidelines, a sample algorithm for the diagnosis of TRALI is shown below 9. 6 Treatment Treatment of TRALI consists mainly of supportive management 4. This may include intubation and mechanical ventilation. About 70% of TRALI patients require mechanical ventilatory support 7. Intravenous fluids may be needed to support pressure once TACO and heart failure have been ruled out 4. Pressors may be necessary for profound hypotension that does not respond to fluids 4. Also, treatment includes the immediate cessation of the transfusion if it has not yet finished 7. Treatment is important not only for the patient in question, but for other patient who may be receiving blood products from a high-risk donor. A TRALI reaction needs to be reported to the transfusion service from which the donor product was obtained so screening tests can be done on the donor plasma. Blood from that donor should never again be given to that patient, but permanent donor deferral has yet to be implemented or shown to be worthwhile 1. Other treatments for TRALI that have been reported include diuretics and steroids. Diuretics have not shown to have a role in the treatment of TRALI and may be contraindicated due to the possible need for pressure support 1. Anecdotal reports of high dose steroids being used to treat TRALI exist and may even have some rationale basis given the immunologic pathophysiology of TRALI, but there have been no good studies showing their efficacy 7. Evidence-based treatment of TRALI is difficult. As of yet, there have been no randomized controlled trials for different treatments of TRALI and the combination of delayed diagnosis with a course that is usually limited to 24-48 hours would make enrolling participants in any such study difficult 4. Prognosis TRALI is a transient condition with a return to pre-transfusion PO2 levels within 48-96 hours for most patients 1. Mortality from TRALI has been approximated to be 510% 1. Although this mortality rate is high and needs to be addressed, it is also hopeful when contrasted to the 40-70% mortality rate associated with the clinically similar ARDS 1 . Also, for those that recover from TRALI, there appears to be no long-term decrease in lung functioning 1. It has been shown that patient prognosis is better when pulmonary infiltrates and hypoxemia resolve quickly 1. TRALI that is antibody-mediated seems to have a more severe course than TRALI due to stored lipids 4. The true effect of TRALI may be difficult to ascertain because patients receiving transfusions are usually in worse condition and the consequences of TRALI may be both primary lung injury and a secondary exacerbation of underlying conditions. Prevention There are three different prevention strategies for TRALI 8: 1. Exclusion of donors believed to be at high risk for causing TRALI 2. Diversion of blood products from high risk donors into pooled components 3. Cellular component processing 7 These management theories are based upon the two proposed pathophysiological mechanisms of TRALI. As stated previously, in the antibody theory, blood products with the highest antibody component such as FFP should have the highest incidence of TRALI 8 . Whereas in the BRM based two-hit hypothesis, blood products with cellular components are needed to cause TRALI 8. Which theory is being addressed drives which preventive actions one thinks is necessary to prevent TRALI 8. Exclusion of Donors Anti-HLA antibodies have been a major focus in the pathogenesis of TRALI and so have also been a major focus in the discussion of preventive strategies. Although antiHLA can be found in anybody, they have a higher prevalence in multiparous women. Sensitization to HLA Class I/II has been correlated with increasing parity. Sensitization rates of 2-8%, 10-15%, and 17-26% have been demonstrated in women with zero, 1-2, and 3+ pregnancies respectively 2. Although multiparous women show increased HLA sensitization rates, this group of donors has not yet been shown to be a significant source of TRALI reactions 5. A granulocyte antibody against human neutrophil antigen-3a has also been implicated. The 3a antigen is ubiquitous, occurring in 89-99% of the population 8 . This antibody has also been associated with multiparity 8. And so multiparous women have become a group focused on for exclusion from the blood pool to theoretically reduce TRALI incidence 8. The reality though is that most blood products from anti-HLA positive women do not cause TRALI 8. Also, reports from two different associations have stated that the exclusion of multiparous women would lead to a decrease in the blood supply of 12% and 33% 8. It has also been said that the exclusion of multiparous women from the blood pool may not be 100% effective, as women may not adequately report ectopic pregnancies and abortions 8. The full consequence of the inclusion or exclusion of this group of women is unknown at this time. Another focus has been on blood donors who have previously received transfusions. Prior blood transfusions have been implicated in high rates of anti-HLA antibodies 8. But exclusion of donors with a history of transfusion would decrease the blood supply even more than the exclusion of multiparous women 8. Product Management Strategies Redirecting blood from donors with a history that suggests high anti-HLA levels like multiparity or a prior transfusion into non-plasma or plasma reduced products has been proposed as a way to reduce TRALI without reducing the donor pool 8. This could be effective because it is not only the presence of antibody, but also the amount of antibody that may play a causative role in TRALI 8. Using high-risk donors for plasma derivatives such as albumin or immune globulin may reduce the amount of antibody present and therefore the incidence of TRALI 8. But there have been reports, though unconfirmed, of pooled products such as IVIG causing TRALI. 8. And one could reasonably expect the incidence of these reactions to rise in pooled components if a significant portion of the high-risk donor pool became diverted into these products. 8 Cellular Component Use and Processing Blood product management strategies based of the two-hit pathogenesis of TRALI are focused more on the reduction of BRMs and leukocytes in cellular components 8. BRMs accumulate over the storage time of the cellular blood products and so some suggest that patients determined to already have a first-hit (ICU, sepsis, etc.) should receive fresher cellular products 8. “Fresher” being described as RBCs that are < 2 weeks old and platelets that are less than 3 days old 8. Washing may also reduce BRMs 8. This involves preparation time and would be impractical for emergency transfusions but could be used for planned transfusions 8. Washing reduces the quantity of cells in blood products and decreases the shelf life of platelets to 4 hours and of RBCs to 24 hours 8. Leukoreduction may also reduce TRALI not only by its effects of reducing WBCs but also by limiting the development of complement-mediated hemolysis 8. A Canadian Transfusion Service was able to show a decrease in the incidence of TRALI after the introduction of leukoreduction 8. Unfortunately, leukoreduction has not significantly reduced the incidence of TRALI in all studies 1. Leukoreduction is mandated in Canada and most of Europe, but not in the United States as of 2005 (I was not able to find clearly stated 2007 guidelines) 1. Summary TRALI is currently the number one cause of transfusion related mortality. The definition, epidemiology and pathogenesis of the syndrome are all still being elucidated. Until there is a better understanding of the cause, there will be limited evidence supporting attempts to manipulate the blood supply to prevent it. The adoption of management strategies without a clear consensus as to their efficacy has been resisted because of fears of reducing the available blood supply 8. References 1. Shander, Aryeh. Understanding the Consequences of Transfusion-Related Acute Lung Injury. Chest 2005; 128; 598-604 2. Triulzi, Darrell. Transfusion-Related Acute Lung Injury: An Update. Hematology 2006;; 497-501 3. Gajic, Ognjen. Pulmonary Edema after Transfusion: How to Differentiate Transfusion-Associated Circulatory Overload from Transfusion-Related Acute Lung Injury. Critical Care Medicine 2006; 34(5 Supplement): S109-113 4. Moore, S. Transfusion-Related Acute Lung Injury (TRALI): Clinical Presentation, Treatment, and Prognosis. Critical Care Medicine 2006; 34(5 Supplement): S114-117 5. Curtis, B. Mechanism of Transfusion-Related Acute Lung Injury (TRALI): Antileukocyte Antibodies. Critical Care Medicine 2006; 34(5 Supplement): S118-123 6. Silliman, C. The Two-Event Model Of Transfusion-Related Acute Lung Injury. Critical Care Medicine 2006; 34(5 Supplement): S124-131 9 7. Barrett, N.A. Transfusion-Related Acute Lung Injury: A Literature Review. Anaesthesia 2006, 61:777-785 8. Mair, D.C. Blood donor and component management strategies to prevent transfusion-related acute lung injury (TRALI). Critical Care Medicine 2006; 34(5 Supplement):S137-143 9. http://www.anaesthesiauk.com/article.aspx?articleid=100902 10