Endoscopic Biopsy of Inflammatory Bowel Disease Dataset
advertisement

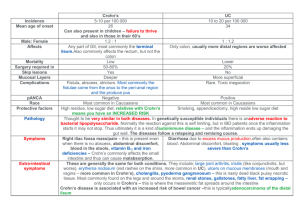
Dataset of Observed Features on Endoscopic Colorectal Biopsies from Normal Subjects and Patients With Chronic Inflammatory Bowel Disease (Crohn’s Disease and Ulcerative Colitis) Dr Simon S Cross Senior Lecturer Department of Pathology University of Sheffield Medical School Beech Hill Road Sheffield S10 2RX, Great Britain E-mail: s.s.cross@sheffield.ac.uk Introduction: Ulcerative colitis and Crohn’s disease are disorders of the digestive tract which extends from the mouth, through the stomach and intestines to the back passage (anus) (Anonymous 1996). Both disorders are grouped under the more generic term ‘chronic idiopathic inflammatory bowel disease’ (CIIBD). CIIBD describes diseases of the bowel which are characterised by acute and chronic inflammation and which have no identified aetiological agent (such as an infective agent). Ulcerative colitis (UC) is restricted to the large intestine which may be inflamed over a variable proportion of its length; if the rectum only is inflamed the condition is described as proctitis. The lining of the intestine becomes red and ulcerated; the inflamed area bleeds readily. For those with extensive colitis there is an increased risk of bowel cancer which can develop in a young person. Crohn’s disease most commonly involves the lower part of the small intestine (ileum) at its junction with the large intestine (‘regional ileitis’) or the small and large intestine, or the colon alone; swelling of the lips and ulceration of the mouth can occur, ulceration and infection around the anus are common. In Crohn’s disease, not only is the lining of the gut swollen and ulcerated, but there is also thickening of the wall of the intestine. The inflammation may spread through the wall to involve neighbouring structures. Local perforation of the wall can lead to widespread or localised infection, or an opening (fistula) on the skin through which intestinal contents emerge. There is an increased risk of cancer, particularly when the large intestine is extensively inflamed. Histopathology is usually recognised as the 'gold standard' for the diagnosis of CIIBD. The two major diagnostic decisions to be made in this area are: 1. Does the subject have CIIBD or not? 2. If the subject does have CIIBD is it Crohn's disease or ulcerative colitis? These decisions have important implications for patient management. The division between CIIBD and not CIIBD determines whether a patient requires long term follow-up or not. The follow-up for CIIBD could include examination of the colon by colonoscopy (a flexible tube passed round the bowel from the anus) on a yearly basis, a procedure that has a small associated mortality, relatively high patient discomfort and a high cost. However lack of follow-up in a patient who has CIIBD could result in a missed opportunity to detect colorectal carcinoma at an earlier, and thus more treatable, stage. The distinction between Crohn's disease and UC becomes important if a patient requires surgical removal of their colon for disease that does not respond to medical therapy. In 1 UC a total colectomy will be curative (since the disease only affects the colon) and the surgical procedure can include formation of an ileoanal pouch providing continence for the patient rather than an ileostomy (a stoma on the abdominal wall with an attached bag). In Crohn's disease such reconstructive surgery is not advised because of the risk of fistula formation between loops of small bowel. Samples for the histopathological diagnosis of CIIBD are taken from the colon at endoscopic examination. These small biopsies (2 mm in diameter) are embedded in paraffin wax and thin sections are stained with haematoxylin and eosin to be examined by light microscopy. The histopathological diagnosis is made subjectively by trained histopathologists. Histopathologists acquire their knowledge and decision-making processes from textbooks and from teaching by more experienced histopathologists, often using a double-headed microscope so teacher and pupil are viewing the same image. In Britain a trainee histopathologist (who will be medically-qualified) is required to have 5 years postgraduate training in recognised laboratories before she/he can take the final examinations of the Royal College of Pathologists and be eligible to become a consultant histopathologist. The diagnostic process in histopathology is poorly-understood but is believed to be a combination of pattern recognition and some form of heuristic logic (Underwood, 1987). The performance of histopathologists in the diagnosis of CIIBD has been investigated by a few published studies but most of these have been carried out in specialist centres for identified studies and so are likely to represent better performance than the overall standard. However these studies produce a sensitivity for the diagnosis of Crohn's disease or ulcerative colitis in the range of 40% to 82% and a specificity for these diagnoses in the range of 73% to 98% (Frei and Morson, 1981; Thompson et al. 1985; Jenkins, 1988; Seldenrijk et al. 1991; Surawicz et al. 1994). There is thus scope for a decision-support system in the histopathological diagnosis of CIIBD to improve the sensitivity and PPV of fully-trained histopathologists and for use in the long training period required for histopathology novices. The Dataset Study population The study population was drawn from large bowel endoscopic biopsies reported in the Department of Histopathology, Royal Hallamshire Hospital, Sheffield between 1990 and 1995 (inclusive) (Dube et al. 1998). Biopsies originating in diverted bowel, rectal stumps or pouches were excluded, as were those with a diagnosis of neoplasm. The diagnosis was confirmed by the finding of typical endoscopy appearances seen on video photographs in the clinical notes, subsequent bowel resection, pattern of disease on radiological investigation or microbiological culture results. In cases without confirmation by subsequent resection specimens this final diagnostic outcome was made with review of the patient's case notes. The biopsies were a mixed population of single distal biopsies and colonoscopic series from initial presentation and follow-up of disease. 2 The observed features The biopsies were examined (blind to all clinical details) by a single experienced observer (SSC) using a computer interface which implements the BSG Guidelines for the Initial Biopsy Diagnosis of Suspected Chronic Idiopathic Inflammatory Bowel Disease (Jenkins et al. 1997) with digitised images representing examples of each histopathological feature (Cross et al. 1997). Some of the features are dichotomous variables, e.g. the presence or absence of mucosal granulomas, whilst others are ordinal categories, e.g. mucin depletion classified into none, mild, moderate or severe. The observed features and their coding are given in table 1. Observation was spread over a period of 9 months with no more than 30 biopsies observed in a single day. Table 1. The observed features and other descriptors in the dataset. Feature Year (=year in which the biopsy was taken) Lab No (=unique laboratory accession number for specified year) Age Sex Active inflammation (subset classifier, not observed feature) Mucosal surface Type Range 0 1 Real integer Binary Binary 14-84 0,1 0,1 Male No Female Yes Flat Irregular Crypt architecture Crypt profiles Increased lamina propria cellularity Mild & superficial increase in lamina propria cellularity Increased lymphoid aggregates in lamina propria? Patchy lamina propria cellularity? Marked & transmucosal increase in lamina propria cellularity Cryptitis extent Cryptitis polymorphs Crypt abscesses extent Crypt abscesses polymorphs Lamina propria polymorphs Epithelial changes Mucin depletion Intraepithelial lymphocytes Subepithelial collagen Lamina propria granulomas Submucosal granulomas Basal histiocytic cells Confirmed diagnosis Method of confirmation Initial pathologists diagnosis Observing pathologists diagnosis Ordinal categorical Real integer Binary Binary 0,1,2,3 Normal 2-7 0,1 No 0,1 No Mild Binary 0,1 No Yes Binary Binary 0,1 0,1 No No Yes Yes Ordinal categorical Ordinal categorical Ordinal categorical Ordinal categorical Ordinal categorical Ordinal categorical Ordinal categorical Binary Binary Binary Binary Binary 0,1,2,3 0,1,2,3 0,1,2,3 0,1,2,3 0,1,2 0,1,2,3 0,1,2,3 0,1 0,1 0,1 0,1 0,1 None None None None Absent Normal Normal Normal Normal Absent Absent Absent Little Few Little Few Focal Flattening Mild Increased Increased Present Present Present Ordinal categorical 0,1,2 3 2 3 Villous projections Moderate Severe Yes Yes Moderate Several Moderate Several Diffuse Degeneration Moderate Marked Many Marked Many Erosion Severe Partitioning of the dataset The Excel file contains 4 worksheets of data that include one with all cases and three subsets for analysis. All cases This worksheet contains all the cases in the study in the order in which they were observed. It is included only as a reference because it contains cases that are not suitable for analysis. These are cases of other diseases, such as mucosal prolapse and melanosis coli, which have small numbers of cases (and so are unlikely to be adequately represented in training and test sets) and which the BSG defined observations do not cover the specific diagnostic features of these diseases (e.g. smooth muscle passing up between crypts in mucosal prolapse, pigment-containing macrophages in the lamina propria in melanosis coli). All IBD&normal This worksheet contains 809 cases of which 165 are normal, 473 UC and 171 Crohn’s disease. These cases are all verified cases of these outcomes with active or inactive inflammation. The cases are in a randomised order and this order should be retained when training and testing. The first 270 cases should be used as a training set, the next 270 cases as an optimisation/verification set and the final 269 cases as a test set. If the methodology does not require a separate optimisation/verification set then the training set can be the first 540 cases. The outcome can be taken as normal v. CIIBD (i.e. UC and Crohn’s combined into a single CIIBD category) or as a tripartite normal v. Crohn’s v. UC outcome. A sequential process could be applied to this dataset with an initial division into normal or CIIBD and then a subsequent division of the CIIBD set into UC or Crohn’s (or inactive and active inflammation and a third division of actively-inflamed cases into UC or Crohn’s). All IBD This worksheet contains 644 cases of which 473 are UC and 171 Crohn’s disease. These cases are all verified cases of these outcomes with active or inactive inflammation. The cases are in a randomised order and this order should be retained when training and testing. The first 215 cases should be used as a training set, the next 215 cases as an optimisation/verification set and the final 214 cases as a test set. If the methodology does not require a separate optimisation/verification set then the training set can be the first 430 cases. The outcome will be Crohn’s v. UC. Active IBD This worksheet contains 370 cases of which 283 are UC and 87 Crohn’s disease. These cases are all verified cases of these outcomes with active or inactive inflammation. The cases are in a randomised order and this order should be retained when training and testing. The first 124 cases should be used as a training set, the next 123 cases as an optimisation/verification set and the final 123 cases as a test set. If the methodology does not require a separate optimisation/verification set then the training set can be the first 247 cases. The outcome will be Crohn’s v. UC. 4 Working practices for analysis: 1. Use the randomised order of data as given in the worksheets 2. Use the partitioning into training, optimisation/verification and test sets as specified above for each set 3. For any process with a variable threshold express the results on a receiver operating characteristic (ROC) curve and use McNemar’s test to compare the area under the curve with other similar processes. Store the coordinates of the ROC curves in clearly labelled Excel worksheets so that these can be imported into graphing programmes 4. At an optimal threshold calculate the sensitivity, specificity, predictive value of a positive result (PV +ve), predictive value of a negative result (PV -ve) and the kappa statistic – all with 95% confidence intervals. Discuss what these optimal thresholds might be with SSC e.g. is sensitivity or specificity most important in separating UC from Crohn’s in biopsies with active inflammation Human performance: All IBD&normal initial pathologists' diagnosis Confirmed outcome Normal 157 Crohn's disease 24 Ulcerative colitis 22 Totals 203 Crohn's disease highly suggestive Crohn's disease suggestive Ulcerative colitis highly suggestive 0 62 0 62 0 14 5 19 0 0 219 219 Ulcerative colitis suggestive CIIBD indeterminate Infective type colitis 0 2 54 56 3 41 146 190 0 0 3 3 Lymphocytic colitis 0 0 0 0 Melanosis coli Inflammation unclassified 2 3 1 27 0 24 3 54 165 171 473 809 Normal Initial pathologists' diagnosis Totals 5 If the CIIBD categories are combined and all other categories combined as not CIIBD this table results: Confirmed outcome Initial Not CIIBD pathologists' diagnosis CIIBD Not CIIBD 162 CIIBD 101 Totals 263 3 165 543 644 546 809 Totals Sensitivity 84% (82-87%) Specificity 98% (96-99%) PV +ve result 99% (98-99%) PV -ve result 62% (56-67%) Kappa 0.68 (0.62-0.73) If the highly suggestive and suggestive of Crohn's disease categories are combined and all other categories combined as not Crohn's disease this table results: Confirmed outcome Initial Not Crohn's disease pathologists' diagnosis Crohn's disease Not Crohn's disease 633 Crohn's disease 95 Totals 728 5 638 76 171 81 809 Totals Sensitivity 44% (37-52%) Specificity 99% (98-99%) PV +ve result 94% (89-99%) PV -ve result 87% (85-89%) Kappa 0.54 (0.46-0.63) If the highly suggestive and suggestive of UC categories are combined and all other categories combined as not UC this table results: Confirmed outcome Not UC UC Initial pathologists' Not UC UC 334 2 200 273 Totals 534 275 diagnosis Totals 336 473 809 Sensitivity Specificity PV +ve result PV -ve result 58% 99% 99% 63% 6 (53-62%) (98-99%) (98-99%) (58-67%) Kappa 0.53 (0.47-0.58) All IBD&normal observing pathologist's diagnosis Confirmed outcome Normal 151 Crohn's disease 64 Ulcerative colitis 90 Totals 305 Crohn's disease highly suggestive 0 15 2 17 Crohn's disease suggestive Ulcerative colitis highly suggestive 0 11 12 23 1 14 138 153 Ulcerative colitis suggestive CIIBD indeterminate Infective type colitis Lymphocytic colitis 0 9 64 73 3 30 108 141 0 3 2 5 0 0 1 1 Melanosis coli Inflammation unclassified 1 9 1 24 1 55 3 88 165 171 473 809 Normal Observing pathologist's diagnosis Totals If the CIIBD categories are combined and all other categories combined as not CIIBD this table results: Confirmed outcome Observing Not CIIBD pathologist's diagnosis CIIBD Not CIIBD 315 CIIBD 87 Totals 402 4 319 403 490 407 809 Totals Sensitivity 82% (79-86%) Specificity 99% (98-99%) PV +ve result 99% (98-99%) PV -ve result 78% (74-82%) Kappa 0.77 (0.73-0.82) 7 If the highly suggestive and suggestive of Crohn's disease categories are combined and all other categories combined as not Crohn's disease this table results: Confirmed outcome Observing Not Crohn's disease pathologist's diagnosis Crohn's disease Not Crohn's disease 624 Crohn's disease 145 Totals 769 14 638 26 171 40 809 Totals Sensitivity 15% (10-21%) Specificity 98% (97-99%) PV +ve result 65% (50-80%) PV -ve result 81% (78-84%) Kappa 0.18 (0.07-0.29) If the highly suggestive and suggestive of UC categories are combined and all other categories combined as not UC this table results: Confirmed outcome Observing Not UC pathologist's diagnosis UC Totals Not UC 312 UC 271 Totals 583 24 336 202 473 226 809 Sensitivity 43% (38-47%) Specificity 93% (90-96%) PV +ve result 89% (85-93%) PV -ve result 54% (49-58%) Kappa 0.32 (0.26-0.38) 8 All IBD initial pathologists' diagnosis Confirmed outcome Crohn's disease 25 Ulcerative colitis 22 Totals 47 Crohn's disease highly suggestive 62 0 62 Crohn's disease suggestive Ulcerative colitis highly suggestive 14 5 19 0 219 219 Ulcerative colitis suggestive CIIBD indeterminate Infective type colitis Melanosis coli 2 54 56 41 146 187 0 3 3 1 0 1 26 24 50 171 473 644 Normal Initial pathologists' diagnosis Inflammation unclassified Totals If the highly suggestive and suggestive of Crohn's disease categories are combined and all other categories combined as not Crohn's disease this table results: Confirmed outcome Initial Not Crohn's disease pathologists' diagnosis Crohn's disease Not Crohn's disease 468 Crohn's disease 95 Totals 563 5 473 76 171 81 644 Totals Sensitivity 44% (37-52%) Specificity 99% (98-99%) PV +ve result 94% (89-99%) PV -ve result 83% (80-86%) Kappa 0.52 (0.44-0.61) 9 If the highly suggestive and suggestive of UC categories are combined and all other categories combined as not UC this table results: Confirmed outcome Initial Not UC pathologists' diagnosis UC Totals Not UC 169 UC 200 Totals 369 2 171 273 473 275 644 Sensitivity 58% (53-62%) Specificity 99% (97-99%) PV +ve result 99% (98-99%) PV -ve result 46% (41-51%) Kappa 0.41 (0.35-0.48) All IBD observing pathologist's diagnosis Confirmed outcome Crohn's disease 64 Ulcerative colitis 90 Totals 154 Crohn's disease highly suggestive Crohn's disease suggestive Ulcerative colitis highly suggestive 15 2 17 11 12 23 14 138 152 Ulcerative colitis suggestive CIIBD indeterminate Infective type colitis 9 64 73 30 108 138 3 2 5 0 1 1 1 24 1 55 2 79 171 473 644 Normal Observing pathologist's diagnosis Lymphocytic colitis Melanosis coli Inflammation unclassified Totals 10 If the highly suggestive and suggestive of Crohn's disease categories are combined and all other categories combined as not Crohn's disease this table results: Confirmed outcome Observing Not Crohn's disease pathologist's diagnosis Crohn's disease Not Crohn's disease 459 Crohn's disease 145 Totals 604 14 473 26 171 40 644 Totals Sensitivity 15% (10-21%) Specificity 97% (96-99%) PV +ve result 65% (50-80%) PV -ve result 76% (73-79%) Kappa 0.16 (0.05-0.28) If the highly suggestive and suggestive of UC categories are combined and all other categories combined as not UC this table results: Confirmed outcome Observing Not UC pathologist's diagnosis UC Totals Not UC 148 UC 271 Totals 419 23 171 202 473 225 644 Sensitivity 43% (38-47%) Specificity 87% (81-92%) PV +ve result 90% (86-94%) PV -ve result 35% (31-40%) Kappa 0.20 (0.13-0.27) 11 Active IBD initial pathologists' diagnosis Confirmed outcome Crohn's disease 0 Ulcerative colitis 0 Totals 0 Crohn's disease highly suggestive 48 0 48 Crohn's disease suggestive Ulcerative colitis highly suggestive 13 4 17 0 143 143 Ulcerative colitis suggestive CIIBD indeterminate Infective type colitis Inflammation unclassified 2 40 42 20 85 105 0 3 3 4 8 12 87 283 370 Normal Initial pathologists' diagnosis Totals If the highly suggestive and suggestive of Crohn's disease categories are combined and all other categories combined as not Crohn's disease this table results: Confirmed outcome Not Crohn's disease Crohn's disease Initial pathologists' Not Crohn's disease Crohn's disease 279 4 26 61 Totals 305 65 diagnosis Totals 283 87 370 Sensitivity 70% (60-80%) Specificity 99% (97-99%) PV +ve result 94% (88-99%) PV -ve result 91% (88-95%) Kappa 0.75 (0.67-0.84) 12 If the highly suggestive and suggestive of UC categories are combined and all other categories combined as not UC this table results: Confirmed outcome Initial Not UC pathologists' diagnosis UC Totals Not UC 85 UC 100 Totals 185 2 87 183 283 185 370 Sensitivity 65% (59-70%) Specificity 98% (95-99%) PV +ve result 99% (97-99%) PV -ve result 46% (39-53%) Kappa 0.45 (0.36-0.54) Active IBD observing pathologist's diagnosis Confirmed outcome Crohn's disease 0 Ulcerative colitis 1 Totals 1 Crohn's disease highly suggestive Crohn's disease suggestive Ulcerative colitis highly suggestive 13 2 15 11 10 21 13 130 143 Ulcerative colitis suggestive CIIBD indeterminate Infective type colitis 8 43 51 25 67 92 3 2 5 Inflammation unclassified 14 28 42 Totals 87 283 370 Normal Observing pathologist's diagnosis 13 If the highly suggestive and suggestive of Crohn's disease categories are combined and all other categories combined as not Crohn's disease this table results: Confirmed outcome Observing Not Crohn's disease pathologist's diagnosis Crohn's disease Not Crohn's disease 262 Crohn's disease 61 Totals 323 21 283 26 87 47 370 Totals Sensitivity 30% (20-40%) Specificity 93% (90-96%) PV +ve result 55% (41-70%) PV -ve result 81% (77-85%) Kappa 0.27 (0.13-0.41) If the highly suggestive and suggestive of UC categories are combined and all other categories combined as not UC this table results: Confirmed outcome Observing Not UC pathologist's diagnosis UC Totals Not UC 66 UC 110 Totals 176 21 87 173 283 194 370 Sensitivity 61% (55-67%) Specificity 76% (67-85%) PV +ve result 89% (85-94%) PV -ve result 38% (30-45%) Kappa 0.27 (0.17-0.37) Discussion of human performance The performance of the initial reporting pathologists is better than that of the observing pathologist despite the fact that the observing pathologist is an experienced specialist gastrointestinal pathologist whereas the initial reporting pathologists were a heterogeneous group of trainee and consultant pathologists without specialism in gastrointestinal pathology. This is likely to be explained by the different environments in which the diagnoses were made. The observing pathologist made his diagnosis with no clinical details about the case whereas the initial reporting pathologists would have taken into account the clinical details written on the histopathology request form. These details may have been very specific, e.g. 'long-standing UC, surveillance colonoscopy', and thus given the clinician's diagnosis to the pathologist before the pathologist had viewed the slides. For the purposes of comparison with statistical methods the performance of the observing pathologists should be taken as the most appropriate benchmark. 14 References: Anonymous (1996) Inflammatory Bowel Disease. London: British Society for Gastroenterology. Cross, S.S., Dube, A.K., Underwood, J.C.E., Pialle, H., Sharkey, A. and Jenkins, D. (1997) A visual image-based data entry system for reporting non-neoplastic endoscopic colorectal biopsies. J.Pathol. 181, 17A(Abstract) Dube, A.K., Cross, S.S. and Lobo, A.J. (1998) Audit of the histopathological diagnosis of non-neoplastic colorectal biopsies: achievable standards for the diagnosis of inflammatory bowel disease. J.Clin.Pathol 51, 378-381. Frei, J.V. and Morson, B.C. (1981) Medical audit of rectal biopsy diagnosis of inflammatory bowel disease. J.Clin.Pathol. 35, 341-344. Jenkins, D. (1988) Computing and histopathology of intestinal inflammation. In: Vicery, F.R., (Ed.) Computers in Gastroenterology, pp. 193-204. London: SpringerVerlag] Jenkins, D., Balsitis, M., Gallivan, S., Dixon, M.F., Gilmour, H.M., Shepherd, N.A., Theodossi, A. and Williams, G.T. (1997) Guidelines for the initial biopsy diagnosis of suspected chronic idiopathic inflammatory bowel disease. The British Society of Gastroenterology Initiative. J.Clin.Pathol. 50, 93-105. Seldenrijk, C.A., Morson, B.C., Meuwissen, S.G., Schipper, N.W., Lindeman, J. and Meijer, C.J. (1991) Histopathological evaluation of colonic mucosal biopsy specimens in chronic inflammatory bowel disease: diagnostic implications. Gut 32, 1514-1520. Surawicz, C.M., Haggitt, R.C., Husseman, M. and McFarland, L.V. (1994) Mucosal biopsy diagnosis of colitis: acute self-limited colitis and idiopathic inflammatory bowel disease. Gastroenterology 107, 755-763. Thompson, E.M., Price, A.B., Altman, D.G., Sowter, C. and Slavin, G. (1985) Quantitation in inflammatory bowel disease using computerised interactive image analysis. J.Clin.Pathol. 38, 631-638. Underwood, J.C.E. (1987) Introduction to Biopsy Interpretation and Surgical Pathology, 2 edn. London: Springer-Verlag. 15