KEY + Name due Wed, Oct 23, 2001
advertisement
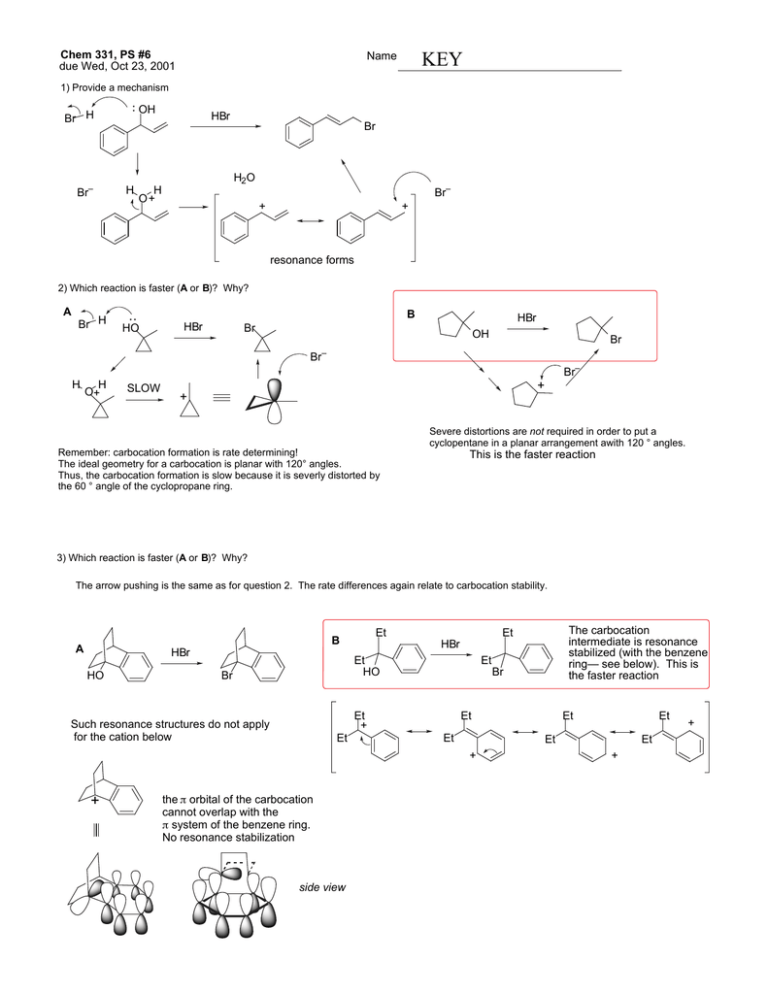
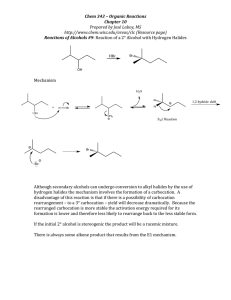
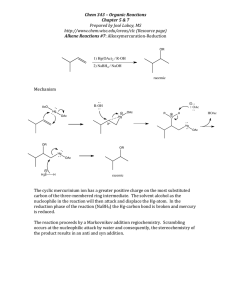
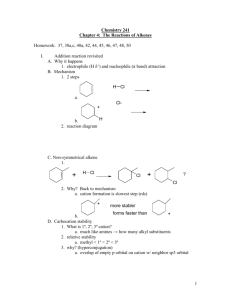
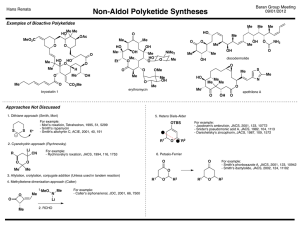
Chem 331, PS #6 due Wed, Oct 23, 2001 Name KEY 1) Provide a mechanism OH Br H Br– HBr Br H2O H H O+ Br– + + resonance forms 2) Which reaction is faster (A or B)? Why? A Br H B HO HBr HBr Br OH Br Br– Br– H H O+ SLOW + + Severe distortions are not required in order to put a cyclopentane in a planar arrangement awith 120 ° angles. Remember: carbocation formation is rate determining! The ideal geometry for a carbocation is planar with 120° angles. Thus, the carbocation formation is slow because it is severly distorted by the 60 ° angle of the cyclopropane ring. This is the faster reaction 3) Which reaction is faster (A or B)? Why? The arrow pushing is the same as for question 2. The rate differences again relate to carbocation stability. Et B A HBr HO Et HO Br Et Br Et + Such resonance structures do not apply for the cation below Et Et Et the π orbital of the carbocation cannot overlap with the π system of the benzene ring. No resonance stabilization side view Et Et Et + + The carbocation intermediate is resonance stabilized (with the benzene ring— see below). This is the faster reaction Et HBr Et + + 4) Provide a detailed explanation Br Me X but Me Br O Me Me H H(eq) Br Me (eq) H E2 elimination can Me occur via an antiperiplanar conformation i.e. both the hydrogen and the bromine are axial H NaOtBu Br NaOtBu H Br(ax) H( ax) the molecule cannot adopt an conformation where Br and H are anti-periplanar. At least one group must be equatorial. – 5) Draw the product of the following reaction. Explain your assignment and provide a mechanism. NaOtBu tBu OAc 13 C12H20O2 21.4 (q) 22.6 (t) 28.9 (t) 29.2 (q, 3 carbons) 32.9 (s) 45.9 (d) 70.5 (d) 128.1 (d) 133.0 (d) 170.9 (s) OAc The most stable conformer has t-Bu in the axial position. Two antiperiplanar E2 eliminations are possible to give structures A and B. – H(ax) H NMR: 0.90 (s, 9H) 1.40 (m, 1H) 1.85 (m, 2H) 2.10 (s, 3H) 2.15 (m, 2H) 5.25 (m, 1H) 5.60 (m, 1H) 5.80 (m, 1H) would expect singlet this is not observed O O– H( ax) tBu OAc (eq) tBu 1 C NMR: H( ax) O CH3 OAc (ax) O A tBu H(ax) OAc tBu O CH3 OAc (ax) B O We need to use the spectral data to pick the right product. We can tell right away by looking at the 13 C NMR that A is not correct— we would expect one of the alkene carbons to be a singlet. The complete assignments are below: 1.85 (m, 2H) 22.6 (t) 28.9 (t) 45.9 (d) 2.15 (m, 2H) 5.80 (m, 1H) 1.40 (m, 1H) 70.5 (d) 170.9 (s) O 29.2 (q, 3 carbons) O 0.90 (s, 9H) CH3 O 32.9 (s) 128.1 (d) 133.0 (d) CH3 21.4 (q) O 5.25 (m, 1H) 5.60 (m, 1H) 2.10 (s, 3H)