Alkenes and alkynes
advertisement

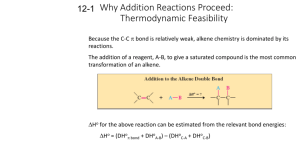
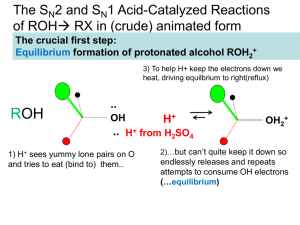
Alkenes and alkynes The chemistry of unsaturation Alkene structure • Unsaturated hydrocarbons – those with one or more double or triple bonds. General formula is CnH2n (for one double bond) • Trigonal planar geometry, sp2 hybridization • Bond angles close to 120º • No rotation about C=C Alkyne structure • One or more triple bonds • Linear geometry • 180º bond angles • sp hybridization • One sigma and two pi bonds H-C≡C-H Ethyne (acetylene) Nomenclature • Position of double bond is given by number of first doubly bonded carbon • 1-butene CH2=CHCH2CH3 • 2-butene CH3CH=CHCH3 • Longest chain always includes double/triple bonds Cis-trans isomerism trans-2-butene E-Z notation cis-2-butene E-Z Notation • Cahn-Ingold sequence priority – priority is given to higher MW substituents • Higher priority substituents on same side – Z isomer (zusammen, or together) Z-2-chloro-2-butene Nomenclature • Higher priority substituents on opposite sides – E isomer (entgegen, against) E-2-chloro-2-butene Nomenclature • Cycloalkenes – double bonded carbons are numbers 1 & 2 4-methylcyclohexene (A number for the double bond is not needed) Nomenclature • Dienes, trienes and polyenes • Prefixes di=2, tri=3, etc. are used to indicate the number of double bonds • Each double bond gets its own location number 3-chloro-1,3-pentadiene Nomenclature 2-methyl-1,4-cyclohexadiene Physical properties • Physical properties of alkenes are similar to those of alkanes • Alkene natural products: Terpenes • Oligomers of isoprene (2-methyl-1,3butadiene) Terpenes • Essential oils – two isoprene units – monoterpenes Terpenes • Sesquiterpenes – three isoprenes • Tetraterpenes (8 isoprenes) b-carotene (precursor to vitamin A) Reactions of alkenes • Addition reactions • Addition of hydrogen halides to form alkyl halides CH2=CH2 + HBr CH3CH2Br • Mechanism – Hydrogen halides are polar – The positive end is attracted to the electrons in the double bond Mechanism of hydrohalogenation • protonation step nucleophile – double bond electrophile – H of HCl 1º (primary) carbocation (unfavorable) Mechanism of hydrohalogenation chloroethane • Carbocation mechanism! Details of Carbocation Mechanisms motion of 1 e- motion of 2 e- • Order of stability of carbocations: 3º>2º>1º • Primary Details of Carbocation Mechanisms • Secondary • Tertiary Details of Carbocation Mechanisms • More substituted carbocations are more stable because alkyl groups are slightly electron donating, and they stabilize the carbocation by diluting the positive charge. • Most stable carbocation possible will be formed Details of Carbocation Mechanisms 2-methyl-2-butene: two possible sites for carbocation formation Tertiary carbocation is always formed – never the secondary Details of Carbocation Mechanisms • Markovnikov’s rule – in hydrohalide addition, the hydrogen adds to the carbon that already has the most hydrogens bonded to it. • The halogen adds to the carbon that has the most carbons attached (the location of the positive charge) Addition of water • Acid catalyzed addition of water to form alcohols Addition of water • Proceeds by a carbocation mechanism • Follows Markovnikov’s rule (“gives the Markovnikov product”) • Mechanism Addition of water protonated ethanol Addition of water ethanol Addition of halogens • Addition of halogens to form vicinal dihalides CH2=CH2 + Br2 BrCH2CH2Br • Carried out in pure reagent, CCl4 or other inert solvent • Additions to cyclic alkenes always give the trans product (“anti” addition) • Discoloration of bromine solutions is a test for alkenes Addition of hydrogen • Addition of hydrogen (reduction) to form alkanes – catalytic hydrogenation • Powdered metal catalyst is used – usually Pd, Pt, Ni, Ru • H2 is used under high pressure Polymerization reactions • Polymers are long chains of identical units called monomers • nCH2=CH2 + initiator (─CH2CH2─)n ethane polyethylene Mechanism of free radical polymerization • Free radicals are species with one unpaired electron • Formed by heterolytic bond cleavage • Initiation Mechanism of free radical polymerization • Propagation • Termination Mechanism of free radical polymerization Polyethylenes • Low density polyethylene (LDPE) • Highly branched, clear, low melting – Made via radical mechanism – Used for packaging, trash bags • High density polyethylene (HDPE) – Linear, opaque, high melting – Made via an ionic mechanism – Used for milk and water jugs, grocery bags, squeezable bottles