CHEMISTRY 163 - Seattle Central College
advertisement
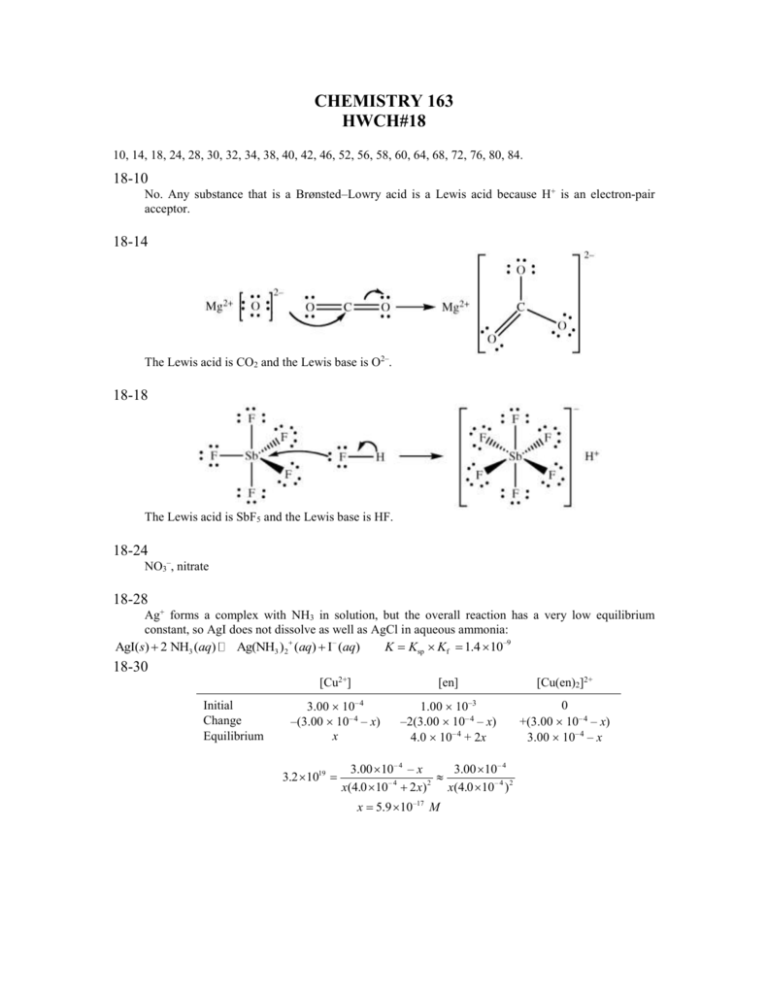
CHEMISTRY 163 HWCH#18 10, 14, 18, 24, 28, 30, 32, 34, 38, 40, 42, 46, 52, 56, 58, 60, 64, 68, 72, 76, 80, 84. 18-10 No. Any substance that is a Brønsted–Lowry acid is a Lewis acid because H+ is an electron-pair acceptor. 18-14 The Lewis acid is CO2 and the Lewis base is O2–. 18-18 The Lewis acid is SbF5 and the Lewis base is HF. 18-24 NO3–, nitrate 18-28 Ag+ forms a complex with NH3 in solution, but the overall reaction has a very low equilibrium constant, so AgI does not dissolve as well as AgCl in aqueous ammonia: AgI(s) 2 NH3 (aq) Ag(NH3 )2 (aq) I – (aq) K Ksp Kf 1.4 10–9 18-30 Initial Change Equilibrium [Cu2+] [en] [Cu(en)2]2+ 3.00 10– 4 –(3.00 10– 4 – x) x 1.00 10–3 –2(3.00 10– 4 – x) 4.0 10– 4 + 2x 0 +(3.00 10– 4 – x) 3.00 10– 4 – x 3.2 1019 3.00 10 – 4 – x 3.00 10 – 4 –4 2 x(4.0 10 2 x) x(4.0 10 – 4 ) 2 x 5.9 10 –17 M 18-32 First, we can assume that all of the Ag+ present is converted to Ag(CN)2– so [Ag(CN)2–] = 4.00 10–5 M. The [CN–] therefore initially is [(4.00 10– 4) – (2 4.00 10–5)] = 3.2 10– 4 M and the amount of Br– in solution is 4.00 10– 4 M. Using an ICE table we can then calculate the amount of Ag + in the solution at equilibrium. To solve this problem for x we will allow AgBr to form as a soluble species, even though we know it to be insoluble. [AgBr] [CN–] [Ag(CN)2–] [Br–] Initial Change Equilibrium 0 +x +x 3.2 10– 4 +2x 4.00 10–5 –x 4.00 10– 4 –x 3.2 10– 4 + 2x 4.00 10–5 – x 4.00 10– 4 – x 4.00 10 – x 4.00 10 x 3.20 10 2 x –5 5.4 108 –4 –4 – x 2 4.00 10 4.00 10 x 3.20 10 –5 –4 –4 2 x 2.9 10–10 M This is a very small amount of AgBr mol 187.77 g 2.9 10–10 0.250 L 1.36 10–8 g AgBr L 1 mol This small amount of AgBr could not be seen in the solution. The contents of the flask appear clear. 18-34 (a) Diamminecopper(I) (b) Tetraaquadihydroxotitanium(IV) (c) Tetraamminediaquanickel(II) 18-38 (a) Ammonium hexacyanocobaltate(III) (b) Chlorobis(ethylenediamine)cobalt(III) nitrate (c) Tetraaquadihydroxoiron(III) chloride 18-40 Ti4+ has the greatest positive charge and is the smallest cation of the group, so we would predict that an aqueous solution of TiCl4 would have the lowest pH. 18-42 (a) As Al3+ reacts with OH– it first forms a precipitate of Al(OH)3: Al3+(aq) + 3 OH–(aq) Al(OH)3(s) Upon further reaction with OH–, the precipitate forms the soluble anion Al(OH) 4– and so the precipitate dissolves to give a clear solution: Al(OH)3(s) + OH–(aq) Al(OH)4–(aq) (b) No, addition of KOH to FeCl3 does not produce the same changes as the addition of KOH to AlCl3. Fe3+ forms an insoluble precipitate with OH–, but does not react further to form a soluble complex ion: Fe3+(aq) + 3 OH–(aq) Fe(OH)3(s) 18-46 No. The solubility of FeCl3 is highest in more acidic solutions because the insoluble Fe(OH) 3 compound formed by Fe3+ hydrolysis reacts with H+ to form Fe3+: Fe(OH)3(s) + 3 H+(aq) Fe3+(aq) + 3 OH–(aq) Because all these acid solutions have different pH, each contains a different [Fe 3+]. 18-52 18-56 18-58 Ti4+ has an electron configuration of [Ar]4s03d 0. Because Ti4+ has no electrons in its d orbitals, no d – d transitions can occur, and the compounds of Ti4+ are colorless. 18-60 The repulsions between the electrons on the ligands and the electrons in the d orbitals of the transition metal cations raise the d orbitals in energy. 18-64 6.626 10 –34 J s 3.00 108 m/s 2.95 10 –7 m or 295 nm 6.74 10 –19 J/ion This wavelength is in the UV region, so the solution is colorless. 18-68 In order to bond to six water ligands the Fe3+ ion must use the 4s, 4p3, and 4d 2 orbitals because electrons occupy the 3d orbitals. The hybridization is then “4s4p34d 2” or sp3d 2 for Fe(H2O)63–. Because the d Z2 and d x2 – y 2 orbitals of Fe(CN)63– are empty, this complex can use the 3d orbitals for bonding to CN–. The hybridization is then “3d 24s4p3” or d 2sp3 for Fe(CN)63–. 18-72 High-spin Fe3+ has 5 unpaired electrons. Rh+ has 2 unpaired electrons. V3+ has 2 unpaired electrons. Low-spin Mn3+ has 2 unpaired electrons. 18-76 After filling each orbital diagram with 8 electrons, we see that Ni(CN)42– is diamagnetic because it has a square-planar geometry and NiCl42– is paramagnetic because it has a tetrahedral geometry. 18-80 No, not all square-planar complexes with two different ligands have geometric isomers. For example, A is a chelating ligand such as ethylenediamine, that ligand occupies only cis positions and if A gives only one isomer of MA2B2. 18-84 H2 N CN CN Cl Cl Cl Cl Ni N H2 H2 N Ni CN CN Cl Cl CN CN Ni N H2 H2 N CN CN Cl Cl CN CN Cl Cl CN Cl Superimposable mirror images Not chiral H2 N N H2 H2 N Ni CN Cl N H2 Ni Ni N H2 H2 N N H2 Superimposable mirror images Not chiral Nonsuperimposable mirror images Chiral









