Semester 1 Exam
advertisement
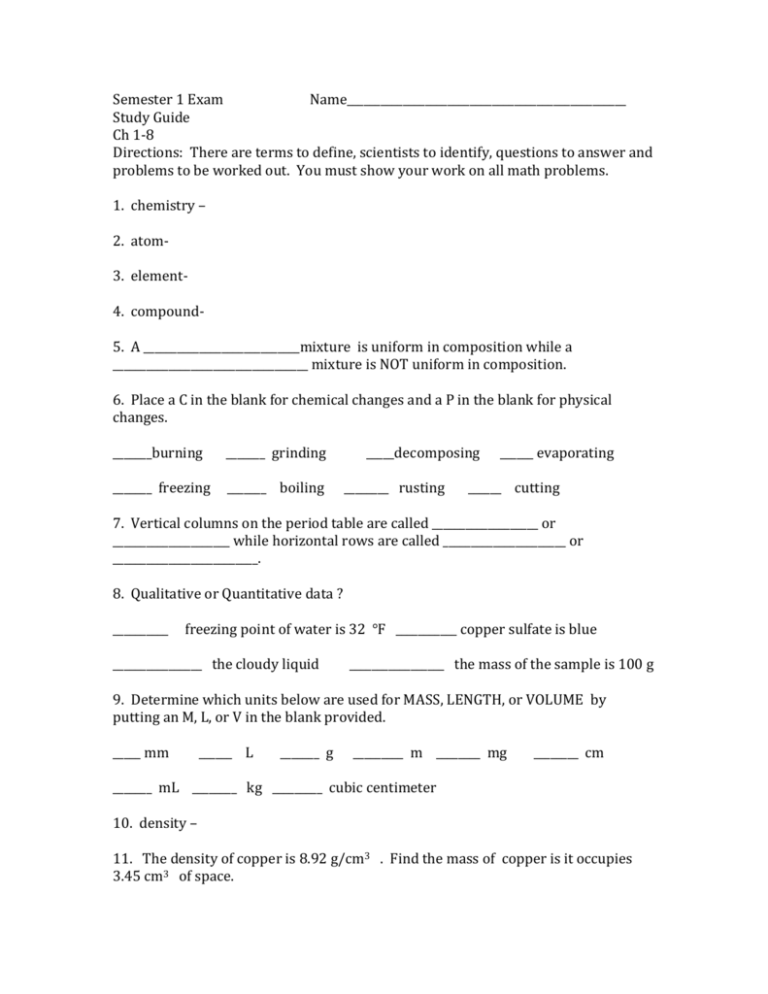
Semester 1 Exam Name__________________________________________________ Study Guide Ch 1-8 Directions: There are terms to define, scientists to identify, questions to answer and problems to be worked out. You must show your work on all math problems. 1. chemistry – 2. atom3. element4. compound5. A ____________________________mixture is uniform in composition while a ___________________________________ mixture is NOT uniform in composition. 6. Place a C in the blank for chemical changes and a P in the blank for physical changes. _______burning _______ grinding _______ freezing _______ boiling _____decomposing ________ rusting ______ evaporating ______ cutting 7. Vertical columns on the period table are called ___________________ or _____________________ while horizontal rows are called ______________________ or __________________________. 8. Qualitative or Quantitative data ? __________ freezing point of water is 32 ℉ ___________ copper sulfate is blue ________________ the cloudy liquid _________________ the mass of the sample is 100 g 9. Determine which units below are used for MASS, LENGTH, or VOLUME by putting an M, L, or V in the blank provided. _____ mm ______ L _______ g _________ m ________ mg ________ cm _______ mL ________ kg _________ cubic centimeter 10. density – 11. The density of copper is 8.92 g/cm3 . Find the mass of copper is it occupies 3.45 cm3 of space. 12. Convert using dimensional analysis. a) How many liters are in 8750 mL ? b) How many grams are in 0.75 kg? 13. Determine the number of significant figures in each of the following: _________ 0.0045 ________ 100.05 _________ 0.0000750 _______ 2.0060 14. Express each in scientific notation. 1000 ________________________________ 0.000430 _____________________________________ 15. Name the scientist who: …developed the atomic theory. _______________________________________ …discovered the electron_________________________________. What piece of equipment did he use to do this?_______________________________________________ …discovered the nucleus? ____________________________________ What was the name of his experiment?______________________________________________________________________ …said that the electron can circle the nucleus only in allowed path, or orbits.__________________________________ 16. Most of the mass of the atom is in the ___________________________ while most of the volume of the atom is the __________________________________________. 17. Isotopes of an atom have the same number of ________________________ and different numbers of ________________________________. 18. Given: An isotope of fluorine has 9 protons, 9 electrons, and 10 neutrons. What is the atomic number?___________________ …mass number? _____________________ 19. ground state – 20. The _______________________________ model of the atom explains the orbitals of electrons as waves. 21. The s orbital has a __________________________________ shape while the p orbitals are shaped like __________________________ 22. How many orbitals are in each? s _______ p _______ d _______ f ______ 23. What formula is used to find the total number of orbitals per energy level?_______________ How many orbitals are on the 3rd energy level?_______________ 24. What formula is used to find the total number of electrons per energy level?________________ How many electrons are on the 4th energy level? ________________ 25. The noble gas notation for sulfur is [Ne] 3s23p4 . In which period __________ and group_______________ is sulfur located? 26. Write the electron configuration for fluorine which has an atomic number of 9. 27. Name the scientist who: … added the Noble Gas group to the periodic table. _______________________________ … arranged the elements according to their chemical and physical properties.________________________________________ 28. Give the names of the following groups on the periodic table. Group 1 __________________________________ Group 2 ____________________________________ Group 17 ________________________________ Group 18 __________________________________ 29. Define ionization energy – 30. Which types of elements tend to have higher ionization energies? metals or nonmetals 31. Define electronegativity 32. Which element has the highest electronegativity?_____________________________ 33. Atomic radii tend to ( increase or decrease ) in a period and atomic radii tend to (increase or decrease in a group. 34. A positive ion is known as a(n) ____________________________ and a negative ion is known as a(n) ____________________________. 35. Define chemical bond36. The electrons that are involved in chemical bonding are known as ____________________________________ electrons. 37. Most elements on the periodic table bond with each other in order to satisfy the __________________________ rule which means__________________________________________ __________________________________________________. 38. Which group does not normally chemically bond with other elements? _______________________________________ because ________________________________________ ________________________________________________. 39. What type of chemical bond is shown in Lewis structures?___________________________ 40. In Lewis structures, how many electrons should surround each nonmetal except hydrogen? _____________ 41. Draw Lewis structures for the following: HF H2O CBr4 42. How many atoms of each element are present in Fe2(SO4)3? 43. What is the formula for the compound formed by sodium ions and sulfide ions? 44. Name the following compounds. a) AlPO4 ________________________________________ b) Fe(ClO3)2 ______________________________________ c) N2O ____________________________________________ d) Ca(NO3)2 ________________________________________ e) As2O5 ___________________________________________ 45. Write chemical formulas for the following. a) dihydrogen monoxide _________________________________ b) cobalt (III) acetate ____________________________________ c) phosphorous pentachloride ______________________________ d) potassium sulfate _________________________________________ 46. Balance the following equation using coefficients. a) _______ FeCl3 + ________ KOH → b) _________ AlF3 + ________ Br2 → _________ Fe(OH)3 + ______ KCl _________ AlBr3 + _______ F2











