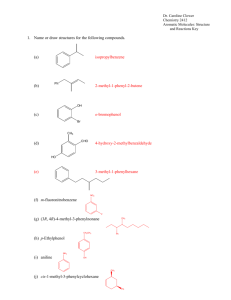
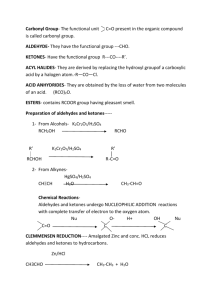
aldehyde and ketone key
advertisement
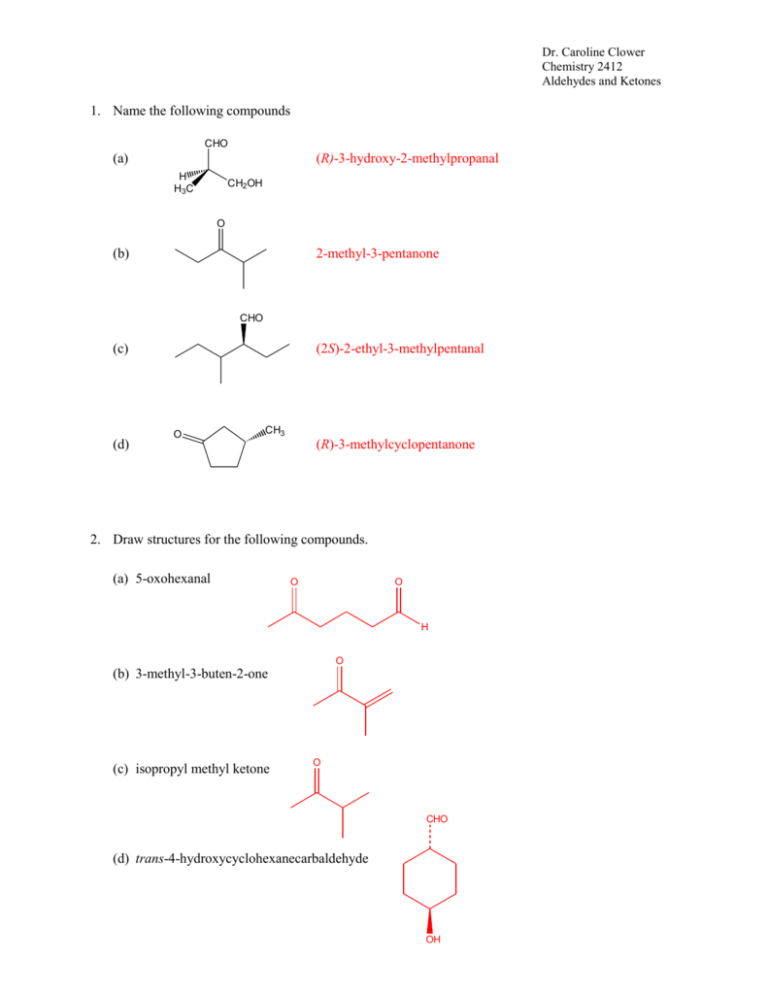
Dr. Caroline Clower Chemistry 2412 Aldehydes and Ketones 1. Name the following compounds CHO (a) (R)-3-hydroxy-2-methylpropanal H H3C CH2OH O (b) 2-methyl-3-pentanone CHO (c) (d) (2S)-2-ethyl-3-methylpentanal O CH3 (R)-3-methylcyclopentanone 2. Draw structures for the following compounds. (a) 5-oxohexanal O O H O (b) 3-methyl-3-buten-2-one (c) isopropyl methyl ketone O CHO (d) trans-4-hydroxycyclohexanecarbaldehyde OH Dr. Caroline Clower Chemistry 2412 Aldehydes and Ketones 3. Identify any nucleophilic and/or electrophilic atoms in the following molecules. (a) Ph3P CH2 E N ON E (b) N (c) E CH3CH2 MgBr 4. Provide structures in the boxes below to complete the following reaction scheme. O HO HC CH C CH 1. NaNH2 H2O, H2SO4 2. Cyclopentanone HgSO4 HO 3. HCl/H2O 5. Give the major organic product(s) for the following reaction. O O HOCH2CH2CH2OH HCl Br Br O C CH 3 Dr. Caroline Clower Chemistry 2412 Aldehydes and Ketones 6. Propose mechanisms for the formation of a hemiacetal using each of the following reagents. O base (a) + CH3CH2OH See Wade p. 813; The base will deprotonate the alcohol to make it a better nucleophile. O (b) + CH3CH2OH H+ See Wade p. 812. The acid will protonante the carbonyl to make it more electrophilic. Dr. Caroline Clower Chemistry 2412 Aldehydes and Ketones 7. Draw the structure of the alcohol and carbonyl compounds formed from acid-catalyzed hydrolysis of the following acetal. CH3CH2 O OCH2CH3 H+ OH O CH3CH2 8. CH3CH2OH + H H2O Provide structures in the empty boxes below to complete the following reaction scheme. H H O + NH3 H+ N H2 H N Ni 9. The following alkene can be synthesized using the Wittig reaction. Show both possible routes, including formation of the phosphonium ylide. O 1. PPh3 CH3CH2Br 2. BuLi CH3CH-PPh3 + O (CH3)2CHBr 1. PPh3 2. BuLi (CH3)2C-PPh3 + H Dr. Caroline Clower Chemistry 2412 Aldehydes and Ketones 10. Show reagents and experimental conditions necessary to bring about each of the following conversion. Et N (a) OH O PCC OH CH2Cl2 H 1. (CH3)2CHMgBr 2. H3O+ OH O N H2CrO4 H2NCH2CH3 H+ O (b) OH OH OH O PCC 1. CH3MgBr CH2Cl2 + 2. H3O OH H H+ OH 1. BH3-THF 2. H2O2, NaOH OH H2CrO4 O