Aldehydes and Ketones
advertisement

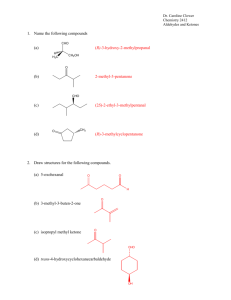
Aldehydes and Ketones Nomenclature Nucleophilic Addition to the Carbonyl Group IUPAC Nomenclature of Aldehydes O O H H O O HCCHCH Base the name on the chain that contains the carbonyl group and replace the -e ending of the hydrocarbon with -al. -al. IUPAC Nomenclature of Aldehydes IUPAC Nomenclature of Aldehydes O O H H 4,4-dimethylpentan al 4,4-dimethylpentanal O O HCCHCH 5-hexen al 5-hexenal or hex-5-enal hex-5-enal O when named as a substituent formyl group 2-phenylpropane dial 2-phenylpropanedial (keep the -e ending before -dial) -dial) C H when named as a suffix carbaldehyde or carboxaldehyde Substitutive IUPAC Nomenclature of Ketones Question O • What is the correct IUPAC name of the aldehyde at the right? • • • • A) B) C) D) CH3CH2 CCH2 CH2CH3 Base the name on the chain that contains the carbonyl group -one. O and replace -e with -one. Number the chain in the direction that gives the lowest number to the carbonyl carbon. H3 C Substitutive IUPAC Nomenclature of Ketones O Functional Class IUPAC Nomenclature of Ketones O 3-hexan one 3-hexanone or Hexan-3-one Hexan-3-one H3 C CH3CHCH2CCH3 O CH3CH2 CCH2 CH2CH3 4-methyl-2pentanone one 4-methyl-2-pentan or 4-methylpentan-2-one 4-methylpentan-2-one O H2 C CHC CH Functional Class IUPAC Nomenclature of Ketones O CH3CH2 CCH2 CH2CH3 ethyl propyl ketone CH2 CH2 List the groups attached to the carbonyl separately in alphabetical order, and add the word ketone. ketone. Question • What is the IUPAC name for the ketone shown below? CH2CCH2CH3 benzyl ethyl ketone O CHC CH CH2CCH2CH3 CH3 4-methylcyclohexanone 4-methylcyclohexanone H2 C O O O CH3CHCH2CCH3 CH3 2,3-dihydroxypropanol 1,2-dihydroxypropanal 2,3-dihydroxypropanal 2,3-propanediol-1-aldehyde CH3CH2 CCH2 CH2CH3 O divinyl ketone • • • • A) B) C) D) 5-ethyl-2-methyl-3-hexanone 2,5-dimethyl-3-heptanone 3,6-dimethyl-5-heptanone 2-ethyl-5-methyl-5-hexanone Question • A compound (C7H14O) has a strong peak in its IR spectrum at 1710 cm-1. Its 1H-NMR spectrum consists of 3 singlets in the ratio 9:3:2 at δ1.0, δ2.1, and δ2.3, respectively. The Carbonyl Group Structure, Bonding Identify this compound. • • • • A) B) C) D) 3-heptanone 2,2-dimethyl-3-pentanone 4,4-dimethyl-2-pentanone 2,4-dimethyl-3-pentanone Carbonyl Group of a Ketone is More Stable than that of an Aldehyde heat of combustion O H 2475 kJ/mol Spread is Greater for Aldehydes and Ketones than for Alkenes O CH3CH2 CH=CH2 2717 kJ/mol H 2475 kJ/mol 2442 kJ/mol cis-CH cis-CH3 CH=CHCH3 2710 kJ/mol trans-CH trans-CH3 CH=CHCH3 2707 kJ/mol O Alkyl groups stabilize carbonyl groups the same way they stabilize carbon-carbon double bonds, carbocations, and free radicals. Heats of combustion of C4 H8 isomeric alkenes O 2442 kJ/mol (CH3 )2C=CH2 2700 kJ/mol Resonance Description of Carbonyl Group •• •• – O •• •• O •• C C + Nucleophiles attack carbon; electrophiles attack oxygen. Synthesis Aldehydes & Ketones Synthesis of Aldehydes and Ketones Question from alkenes A number of reactions already studied provide efficient synthetic routes to aldehydes and ketones. ketones. • What combination of reagents will transform 1-butyne into 2butanone? ozonolysis from alkynes hydration (via enol) from arenes • • • • A) B) C) D) 1. O3 2. H2O, Zn K2Cr2O7, H2SO4 H2SO4, HgSO4 OsO4 (cat), (CH3)3COOH, (CH3) 3OH, HO- Friedel-Crafts acylation from alcohols oxidation Example Reduction & Oxidation aldehydes from carboxylic acids O R C Benzaldehyde from benzoic acid O R OH 1. LiAlH4 2. H2O C H PCC or PDC, CH2 Cl2 RCH2OH O O COH CH 1. LiAlH4 2. H2O CH2OH (81%) Reduction Grignard & Oxidation aldehydes from carboxylic acid esters O R C 1. DIBAL-H 2. H2O Ketones from aldehydes O O (Diisobutyl aluminum hydride) OR’ OR’ (83%) PCC or PDC CH2Cl2 R C or from nitriles R-CN with DIBAL-H H R 1. R'MgX 2. H3O+ C O R H OH RCHR' RCHR' C R' PCC or PDC, CH2 Cl2 Example Question 3-heptanone from propanal • Which combination of reagents will produce 2-hexanone as the major organic product? • • • A) 2-hexanol + PCC in CH2Cl2 B) 1-hexene + H2O, H2SO4, HgSO4 C) pentanal + methylmagnesium bromide followed by H3O+ • D) All of the above (a-c) will produce 2-hexanone as the major product. O CH3CH2 C O CH3CH2 C(CH2)3 CH3 H (57%) 1. CH3 (CH2 )3MgX 2. H3O+ H2 CrO4 OH CH3CH2 CH(CH CH(CH2 )3 CH3 Question • An alcohol with a molecular formula C7H16O was treated with chromic acid. The product produced 4 signals in its 13C-NMR spectrum: one at 210 ppm and 3 others below 50 ppm. Identify the ketone. • A) 4-heptanone • B) 2,4-dimethyl-3-pentanone • C) 4,4-dimethyl-2-pentanone • D) 5-methyl-3-hexanone Reactions of Aldehydes and Ketones Reactions of Aldehydes and Ketones Question • Which compound will be isolated from the synthetic sequence shown below? • A) B) • C) D) Reduction of C=O to CH2 Clemmensen reduction Wolff-Kishner reduction Reduction of C=O to CHOH Addition of Grignard and organolithium reagents Question Aldehydes and ketones react with nucleophiles to form addition products: nucleophile addition reactions • When a nucleophile encounters a ketone, the site of attack is • A) the carbon atom of the cabonyl. • B) the oxygen atom of the carbonyl. • C) both the carbon and oxygen atoms, with equal probability. • D) No attack occurs--ketones do not react with nucleophiles. Hydration of Aldehydes and Ketones C Hydration of Aldehydes and Ketones •• H2 O •• HO •• C •• O •• H Equilibrium Constants and Relative Rates of Hydration Substituent Effects on Hydration Equilibria C=O hydrate K % Relative rate CH2=O CH2 (OH)2 2300 >99.9 2200 compared to H CH3CH=O CH3 CH(OH)2 1.0 50 1.0 electronic: alkyl groups stabilize reactants (CH3)3 CCH=O (CH3)3CCH(OH)2 0.2 17 0.09 (CH3)2 C=O (CH3)2C(OH)2 0.14 0.0018 steric: alkyl groups crowd product OH O + H2 O C R O •• R R' C OH R' 0.0014 Equilibrium Can Favor a Hydrate Substituent Effects on Hydration Equilibria (But not very often) OH O Only when the carbonyl group is destabilized + H2 O C • alkyl groups stabilize C=O C R R R R OH • electron-withdrawing groups destabilize C=O R = CH3: K = 0.0014 R = CF3: K = 22,000 Mechanism of Hydration (base) Mechanism of Hydration (base) Step 1: H – •O• • •• • Step 2: + C •• O •• HO •• •• C •• – O •• •• HO •• •• •• •• C OH •• Mechanism of Hydration (acid) Step 1: O •• •• + H H – + •• O •• O •• •• •• Mechanism of Hydration (acid) Step 2: H C •• H H HO •• – O •• C •• O• + • H C + OH •• H + H •O• • • •O• • • H H + C + OH H •O • + •• H C •• OH •• Mechanism of Hydration (acid) Question Step 3: H •• +O • Which one of the compounds below has the fastest hydration rate? H H C H H • A) B) • C) D) •• H + •• •O • •• O H •O • + •• OH H •• C •• OH •• Cyanohydrin Formation Cyanohydrin Formation C •N • O •• + HCN •• Cyanohydrin Formation C •• O C •• Cyanohydrin Formation H •N • – C •• C O •• •• •N • C C •• – O •• •• H O •• + H H •N • C C •• O •• H • O •• • H H Example Example Cl Cl Cl O NaCN, water Cl CH O OH NaCN, water CH3CCH3 then H2 SO4 CHCN then H2SO4 OH CH3CCH3 CN (77-78%) 2,4-Dichlorobenzaldehyde cyanohydrin (100%) Acetone cyanohydrin is used in the synthesis of methacrylonitrile Some reactions of aldehydes and ketones progress beyond the nucleophilic addition stage Acetal formation Acetal Formation Imine formation Enamine formation Compounds related to imines The Wittig reaction Recall Hydration of Aldehydes and Ketones R C O •• •• R' HOH R •• HO •• C R' •• O •• H Alcohols Under Analogous Reaction with Aldehydes and Ketones Hemiacetal reacts further in acid to yield an acetal R R •• R"O O •• C •• •• •• C •• R' R' ROH, ROH, H+ R"OH R"OH R •• R"O C •• R •• O •• H Product is called a hemiacetal. hemiacetal. •• R"O •• R' • The structure shown at the right would be best classified as a(n) A) B) C) D) •• C O •• Product is called a hemiacetal. hemiacetal. H R' Question • • • • Product is called an acetal. acetal. OR acetal. hemiacetal. hydrate. cyanohydrin. Example O CH + 2CH3CH2 OH HCl CH(O CH(OCH2 CH3)2 + H2O Benzaldehyde diethyl acetal (66%) Diols Form Cyclic Acetals In general: O CH3(CH2)5CH + HOCH2 CH2OH benzene p-toluenesulfonic acid H2 C CH2 O O + H2 O C (81%) H (CH2 )5CH3 Position of equilibrium is usually unfavorable for acetal formation from ketones. ketones. Important exception: Cyclic acetals can be prepared from ketones. ketones. Example Question O C6 H5CH2 CCH3 + • What is the product of the reaction between benzaldehyde and 1,3propanediol? HOCH2 CH2OH benzene p-toluenesulfonic acid (78%) H2 C CH2 O O • A) B) • C) D) + H2 O C CH3 C6 H5CH2 Hydrolysis of Acetals OR" R C O C R' + H2O R OR" mechanism: + R' 2R"O 2R"O H Acetals as Protecting Groups reverse of acetal formation; hemiacetal is intermediate application: aldehydes and ketones can be "protected" as acetals. acetals. Example The conversion shown cannot be carried out directly... O CH3CCH2CH2 C CH 1. NaNH2 2. CH3 I O CH3CCH2CH2 C CCH3 because the carbonyl group and the carbanion are incompatible functional groups. O CH3CCH2CH2 C C: – Example: Protect Strategy O CH3CCH2CH2 C 1) protect C=O CH + HOCH2 CH2OH benzene p-toluenesulfonic acid 2) alkylate 3) restore C=O H2 C CH2 O O C CH2CH2 C CH3 Example: Alkylate H2 C Example: Deprotect CH2 H2 C O O O C CH2CH2 C CH3 CH3 O O CH2CH2 C HOCH2 CH2OH C CH2CH2 C CH What reagent and/or reaction conditions would you choose to bring about the following conversion? A) B) C) D) H2 O 1. LiAlH4; 2. H2O H2O, H2SO4, heat H2O, NaOH, heat PCC, CH2Cl2 CCH3 HCl O Question • • • • O CH2 CH3 • CH2 C CCH3 H2 C 1. NaNH2 2. CH3 I CH + CH3CCH2CH2 C (96%) CCH3