ORGANIC CHEMISTRY
advertisement

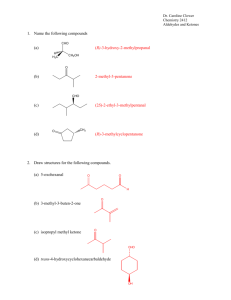
ALDEHYDES AND KETONES Both aldehydes and ketones contain a carbon atom that is joined by a double bond to an oxygen atom. O || The - C- unit is called a CARBONYL group. In the case of an aldehyde, the carbon atom of the carbonyl group is bonded to a hydrogen atom. In a ketone, the carbon atom of the carbonyl group is “sandwiched” between two other carbon atoms. The generic formula for O O || || aldehydes is thus: R—C—H and for ketones is R—C—R’ where R and R’ are alkyl groups. The condensed formulas are R–CHO and R-CO-R’ respectively. THE NAMING OF ALDERYDES AND KETONES The naming of aldehydes and ketones begins by choosing the longest chain containing the carbonyl group to obtain the base name. The ending “e” is removed from the base name and replaced by the suffix “al” in the case of aldehydes and “one” in the case of ketones. Since the carbonyl group of an aldehyde is always at one end of the main chain, its position does not require a number. However, for ketones, we use a number to show the location of the carbonyl group (starting the count from the end closest to the carbonyl group). Examples: CH3 O O CH3 | || || | CH3-CH-CH2-C-H CH3 – C – CH2 – CH – CH3 3-methylbutanal 4-methyl-2-pentanone EXERCISE: 1. Name the following using IUPAC system: CH3 O | || a) CH3 – CH – CH2 – CH2 – C – CH3 CH3 O | || CH3 – C – C -H b) | CH3 2. Draw the structural formula for: a) 3-pentanone b) butanal c) 2-methylpentanal d) 2,4-dimethyl-3-hexanone PROPERTIES OF ALDEHYDES AND KETONES 1) The carbonyl group is quite polar. 2) Due to the polarity of the carbonyl group, aldehydes and ketones have melting points and boiling points HIGHER than ethers having the same number of carbon atoms. However, the boiling points and the melting points of aldehydes and ketones are LOWER than those of alcohols having the same number of carbon atoms. This is because there is no HYDROGEN BONDING in aldehydes and ketones. 3) The lower members of the aldehydes and ketones series are miscible with water. REACTIONS OF ALDEHYDES AND KETONES 1) Both aldehydes and ketones can be reduced to alcohols. One reducing agent that can reduce them is hydrogen gas in the presence of platinum as a catalyst: O || Pt CH3 – C – H + H2 CH3 – CH2 - OH ethanal ethanol O OH || Pt CH3 – C – CH2 – CH2 – CH3 + H2 | CH3 – CH – CH2 – CH2 – CH3 2-pentanone 2-pentanol 2) Aldehydes can be oxidized easily by a variety of oxidizing agents to CARBOXYLIC ACIDS, e.g.; acidified potassium dichromate, K2Cr2O7. O O || || CH3 – C – H ethanal + Cr2O72- + H1+ CH3 – C – OH + Cr3+ + H2O ethanoic acid 3) Ketones resist oxidation. Questions 1. Write an equation for each of the following reactions: a) Balance the reaction between methanal and acidified Cr2O72- ions. Hint: This reaction is a redox reaction. Combine the following two half reactions to obtain a balanced equation. The oxidation half-reaction: HCHO Methanal HCOOH methanoic acid The reduction half-reaction: Cr2O72- —> Cr3+ b)The reaction between propanal and hydrogen gas over platinum as a catalyst. c) The oxidation of 2-methylpropanal with acidified potassium dichromate ions. 2. Which alcohol would you oxidize to prepare a) pentanal and b) 3-pentanone. Write a balanced half reaction for each reaction. a) b)