B. Polarity of Bonds p.18 Difference in electronegativity can be used
advertisement
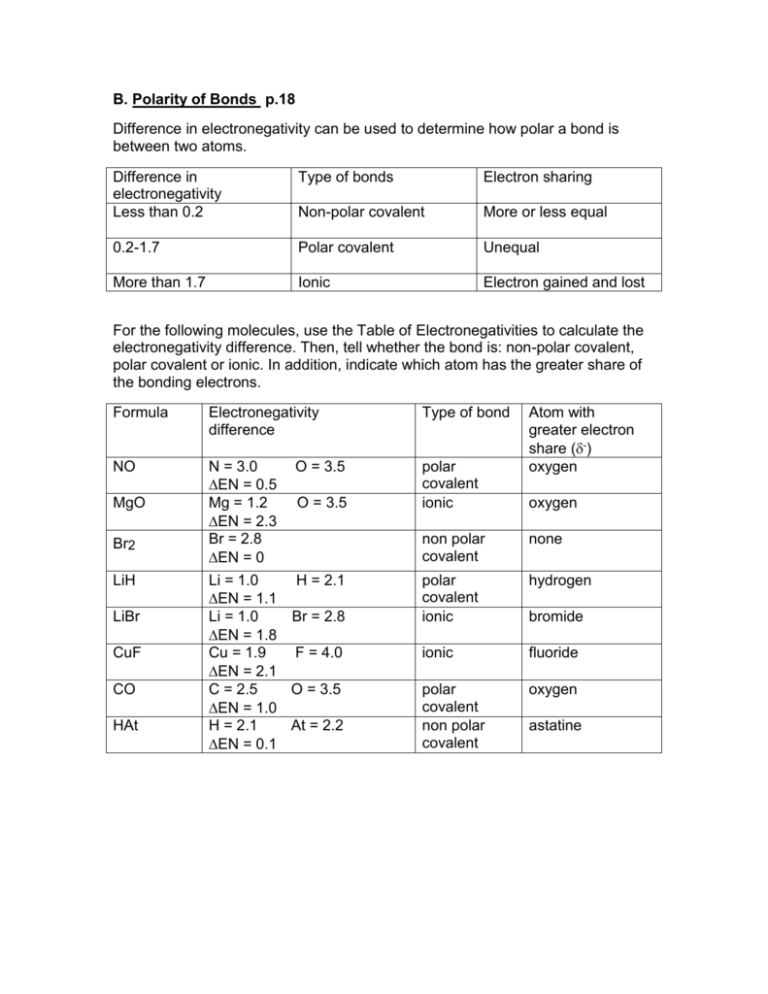
B. Polarity of Bonds p.18 Difference in electronegativity can be used to determine how polar a bond is between two atoms. Difference in electronegativity Less than 0.2 Type of bonds Electron sharing Non-polar covalent More or less equal 0.2-1.7 Polar covalent Unequal More than 1.7 Ionic Electron gained and lost For the following molecules, use the Table of Electronegativities to calculate the electronegativity difference. Then, tell whether the bond is: non-polar covalent, polar covalent or ionic. In addition, indicate which atom has the greater share of the bonding electrons. Formula Electronegativity difference Type of bond NO N = 3.0 O = 3.5 EN = 0.5 Mg = 1.2 O = 3.5 EN = 2.3 Br = 2.8 EN = 0 Li = 1.0 H = 2.1 EN = 1.1 Li = 1.0 Br = 2.8 EN = 1.8 Cu = 1.9 F = 4.0 EN = 2.1 C = 2.5 O = 3.5 EN = 1.0 H = 2.1 At = 2.2 EN = 0.1 polar covalent ionic MgO Br2 LiH LiBr CuF CO HAt Atom with greater electron share (-) oxygen oxygen non polar covalent none polar covalent ionic hydrogen ionic fluoride polar covalent non polar covalent oxygen bromide astatine