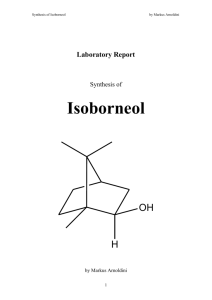
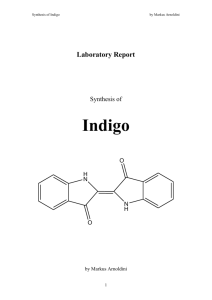
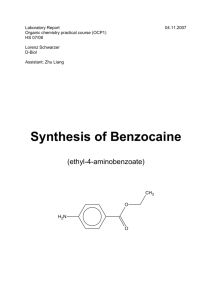
Laboratory Report
advertisement

Synthesis of Triphenylmethanol by Markus Arnoldini Laboratory Report Synthesis of Triphenylmethanol OH by Markus Arnoldini 1 Synthesis of Triphenylmethanol 1. by Markus Arnoldini Method Grignard reaction. 2. Reaction Equation O Br + 3. + Mg + OH H2O Mechanism first step: Br + Mg - Mg - Mg Br + Mg - Mg - Mg + Br + Mg - Mg - Mg MgBr 2 + 2 Mg + Br Synthesis of Triphenylmethanol by Markus Arnoldini second step: O O2Et EtO2 Mg Br + Ligandchange O Mg Br O2Et Nucleophilic Attack MgBr Et2O OEt2 Et2O O Mg O Mg O2Et EtO2 Mg + Br Et2O BrH Mg Br Br OEt2 Ligandchange OEt2 OHMg OEt2 third step: OMgBr OH H + H2O 3 MgBr(OH) Synthesis of Triphenylmethanol 4. by Markus Arnoldini Physical properties of the substances Magnesium (slivers) [1] molar weight density melting point boiling point refraction index WGK GK (CH) R-phrases Mg S-phrases 24.31 g/mol 1.75 g/cm3 (20 ºC) 651 ºC 1107 ºC nwg R 11 (Highly flammable) R 15 (Contact with water liberates extremely flammable gases) S 7/8 (Keep container tightly closed and dry) S 43 (In case of fire use sand. Never use water) Iodine (crystal) [1] molar weight density melting point boiling point refraction index WGK GK (CH) R-phrases I2 S-phrases 253.81 g/mol 4.93 g/cm3 (20 ºC) 114 ºC 185 ºC 1 2 R 20/21 (Harmful by inhalation and in contact with skin) - Sodium Hydrogencarbonate [1] O C HO O Na molar weight density melting point boiling point refraction index WGK GK (CH) R-phrases S-phrases 4 84.01 g/mol 2.22 g/ cm3 270 °C 1 5 - Synthesis of Triphenylmethanol by Markus Arnoldini Diethylether (anhydrous) [1] molar weight density melting point boiling point refraction index WGK GK (CH) R-phrases O S-phrases 74.12 g/mol 0.71 g/cm3 -116.3 ºC 34.6 ºC (1013 hPa) 1 4 R 12 (Extremely flammable) R 19 (May form explosive peroxides) R 22 (Harmful if swallowed) R 66 (Repeated exposure may cause skin dryness or cracking) R 67 (Vapours may cause drowsiness and dizziness) S 9 (Keep container in a well-ventilated place) S 16 (Keep away from sources of ignition - No smoking) S 29 (Do not empty into drains) S 33 (Take precautionary measures against static discharges) Benzophenone [1] O molar weight density melting point boiling point refraction index WGK GK (CH) R-phrases S-phrases 5 182.22 g/mol 1.1 g/cm3 (25 ºC) 47 - 49 ºC 304 - 306 ºC 2 4 R 50/53 (Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment) S 61 (Avoid release to the environment. Refer to special instructions / Safety data sheets) Synthesis of Triphenylmethanol by Markus Arnoldini NaSO4 [1] molar weight density melting point boiling point refraction index WGK GK (CH) R-phrases S-phrases NaSO4 142.04 g/mol 2.70 g/cm3 (20 ºC) 888 ºC 1 5 - Hydrochloric Acid (conc.) [1] molar weight density melting point boiling point refraction index WGK GK (CH) R-phrases HCl S-phrases 36.46 g/mol ~ 1.19 g/cm3 (20 ºC) 1 2 R 34 (causes burns) R 36 (irritates eyes) R 37 (irritates respiratory system) R 38 (irritates skin) S 26 (in case of contact with eyes rinse with a lot of water and seek medical help) Phenylbromide [1] Br molar weight density melting point boiling point refraction index WGK GK (CH) R-phrases 157.02 g/mol 1.49 g/cm3 (20 ºC) -31 ºC 156 ºC 2 3 R 10 (Flammable) R 38 (Irritating to skin) R 51/53 (Toxic to aquatic organisms, may cause long-term adverse effects in aquatic environments) S-phrases S 61 (Avoid release to the environment. Refer to special instructions / Safety data sheets) 6 Synthesis of Triphenylmethanol by Markus Arnoldini Triphenylmethanol [1] OH molar weight density melting point boiling point refraction index WGK GK (CH) R-phrases S-phrases 5. 260.34 g/mol 160-163 °C 360 ºC - Experimental accomplishment 0.9 g of magnesium slivers, one I2 crystal and 20 mL of anhydrous Et2O were put in a 250 ml three-neck round bottom flask with a reflux cooler and a dropping funnel. A solution of 3.5 mL (5.215 g = 0.03 mol) of phenylbromide in 40 mL of anhydrous Et2O was put into the dropping funnel. Then 5-7 mL of the PhBr solution were added dropwisely while stirring. Because nothing happened first, the flask was heated in a water bath to 35 °C and the ether started boiling. At that point the bath was removed. The remaining PhBr solution was added dropwisely and after that the flask was heated again with a water bath to around 35 °C for 15 min. While the flask was heated, a solution made of 4.3 g of benzophenone in 40 mL of anhydrous Et2O was prepared and put into the dropping funnel. After the aforesaid 15 min the solution was added slowly to the reaction mixture, was kept another 5 min at 35 °C and was then cooled down to room temperature. At this point a 6 M solution of HCl was added drop by drop until a pH of about 1 was reached. The mixture was then put in a extraction funnel and the two phases were separated. The water phase was then washed two times with 50 mL of hexane and after every washing step the organic phase was collected together with the organic phase of the very first separation. The collected organic phases were then washed with saturated solutions of NaHCO3 and NaCl and then dried over NaSO4. The Et2O was then removed with a rotovap, and the resulting solid was dissolved in Et2O and purified by column chromatography. An amount of 6.4 g (0.024 mol) of product was received. For the calculation of the yield the molar amount of received product was divided by the molar amount of reactants used, and then multiplied by 100. This calculation yields a percentage of 80 %: 0.024mol 100% 80% 0.03mol 7 Synthesis of Triphenylmethanol 6. by Markus Arnoldini Experimental setup Calciumchloride duct Reflux-cooler Thermometer drop flask three-neck round bottom flask LaboB ib© 300 50 AN AN AUS AUS 1500 0 U/min 250 100 o 500 C 200 150 1000 750 Seperatory funnel 600 Beaker to collect water phase 800 mL 400 200 8 Synthesis of Triphenylmethanol 7. by Markus Arnoldini Analytical results The chromatography yielded two different fractions of product, but the measurement of melting points and IR-spectra indicated that the second fraction was the dimer product. Melting point: 156 – 160 °C Peaks in IR Spectrum (in cm-1): 3300-3600: 3000-3100: ~1600: 970-1050: OH CH (aromatic) aromatic ring C-O 8. Sources [1] [2] http://ch.chemdat.info/mda/ch/de/ meanings of R-phrases: http://www.chemie.fu-berlin.de/chemistry/safety/r-saetze_en.html meanings of S-phrases: http://www.chemie.fu-berlin.de/chemistry/safety/s-saetze_en.html [3] 9