Laboratory Report
advertisement

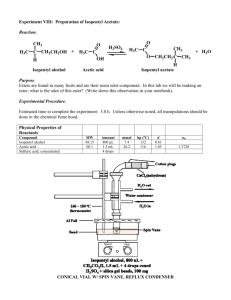
Synthesis of Isopentyl Acetate by Markus Arnoldini Laboratory Report Synthesis of Isopentyl Acetate O O by Markus Arnoldini 1 Synthesis of Isopentyl Acetate 1. by Markus Arnoldini Method Esterification of glacial acetic acid and isopentyl alcohol, using sulfuric acid as catalyst. 2. Reaction Equation O O H2SO4 -H2O + OH 3. O HO Mechanism HSO4 H2SO4 O OH OH + OH OH OH HO HO HO HO O H HO O H2SO4 HSO4 HSO4 OH HO -H2O H 2O O O HSO4 O O 2 Synthesis of Isopentyl Acetate 4. by Markus Arnoldini Physical properties of the substances Isopentyl alcohol [1] molar weight density melting point boiling point refraction index WGK GK (CH) R-phrases HO S-phrases 88.15 g/mol 0.81 g/cm3 (20 ºC) -117 ºC 131 ºC (1013 hPa) 1 R 10 (Flammable) R 20 (Harmful by inhalation) R 37 (Irritating to respiratory system) R 66 (Repeated exposure may cause skin dryness or cracking) S 46 (If swallowed, seek medical advice immediately and show this container or label) Glacial acetic acid [1] molar weight density melting point boiling point refraction index WGK GK (CH) R-phrases O OH S-phrases 3 60.05 g/mol 1.05 g/cm3 (20 ºC) 17 ºC 116 - 118 ºC (1013 hPa) 1.37 (20 ºC) 1 3 R 10 (Flammable) R 35 (Causes severe burns) S 23 (Do not breathe fumes) S 26 (In case of contact with eyes, rinse immediately with plenty of water and seek medical advice) S 45 (In case of accident or if you feel unwell, seek medical advice immediately. Show the label where possible.) Synthesis of Isopentyl Acetate by Markus Arnoldini Sulfuric acid (5N) [1] molar weight density melting point boiling point refraction index WGK GK (CH) R-phrases S-phrases H2SO4 1.15 g/cm (20 ºC) 1 2 R 35 (Causes severe burns) S 26 (In case of contact with eyes, rinse immediately with plenty of water and seek medical advice) S 30 (Never add water to this product) S 36/37/39 (Wear suitable protective clothing, gloves and eye/face protection) S 45 (In case of accident or if you feel unwell, seek medical advice immediately. Show the label where possible.) 3 Sodium Hydrogencarbonate [1] O C HO O Na molar weight density melting point boiling point refraction index WGK GK (CH) R-phrases S-phrases 84.01 g/mol 2.22 g/ cm3 270 °C 1 5 - molar weight density melting point boiling point refraction index WGK GK (CH) R-phrases S-phrases 120.37 g/mol 2.66 g/cm3 (20 ºC) 1 - Magnesium sulfate [1] MgSO4 4 Synthesis of Isopentyl Acetate by Markus Arnoldini Isopentyl acetate [1] O O molar weight density melting point boiling point refraction index WGK GK (CH) R-phrases S-phrases 5. 130.19 g/mol 0.87 g/cm3 (20 ºC) -78 ºC 141 ºC 1 5 R 10 (Flammable) R 66 (Repeated exposure may cause skin dryness or cracking) S 23 (Do not breathe fumes) S 25 (Avoid contact with eyes) Experimental accomplishment 7.5 ml of isopentyl alcohol were put in a 100 ml three neck round bottom flask and 10 ml of glacial acetic acid were added. While stirring, 2 ml of H2SO4 were added drop by drop to the reaction mixture, which was then brought to reflux at 160 °C for 1 h. In this time, the solution turned dark brown. Then it was cooled down to room temperature and washed first with distilled water, then with saturated solutions of NaHCO3 and NaCl and the organic phase was then dried over MgSO4. An amount of 2.788 g pure isopentyl acetate was received by the following distillation. For the calculation of the yield the molar amount of received product was divided by the molar amount of reactants used, and then multiplied by 100. This calculation yields a percentage of 30.435 %: 0.021mol 100% 30.435% 0.069mol 5 Synthesis of Isopentyl Acetate 6. by Markus Arnoldini Experimental setup Calciumchloride duct Reflux-cooler drop flask with H2SO4 Thermometer three-neck round bottom flask LaboB ib© 300 50 1500 AN AN AUS AUS 1000 100 o 0 U/min 250 500 C 200 150 750 Seperatory funnel 600 Beaker to collect water phase 800 mL 400 200 6 Synthesis of Isopentyl Acetate 7. by Markus Arnoldini Analytical results Refraction index at room temperature: 1.404 Peaks in IR Spectrum (in cm-1): 2850-3000: 1700-1780: 1420-1480: 1350-1400: 1200-1280: C-H stretch, aliphatic C=O CH2 CH3 C-O 8. Sources [1] [2] http://ch.chemdat.info/mda/ch/de/ meanings of R-phrases: http://www.chemie.fu-berlin.de/chemistry/safety/r-saetze_en.html meanings of S-phrases: http://www.chemie.fu-berlin.de/chemistry/safety/s-saetze_en.html [3] 7