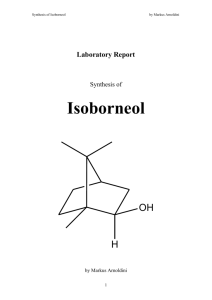
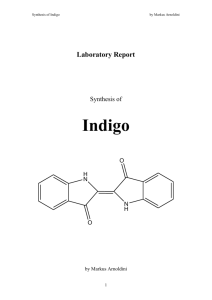
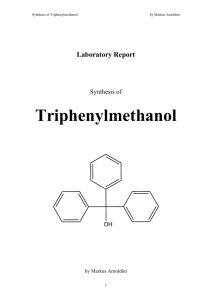
Laboratory Report
advertisement

Synthesis of 9,10,11,15-tetrahydro-9,10[3’,4’]-furanoanthracen-12,14-dione by Markus Arnoldini Laboratory Report Synthesis of 9,10,11,15-tetrahydro-9,10[3’,4’]furanoanthracen-12,14-dione O O O by Markus Arnoldini 1 Synthesis of 9,10,11,15-tetrahydro-9,10[3’,4’]-furanoanthracen-12,14-dione 1. by Markus Arnoldini Method Diels-Alder-Reaction (4+2 cycloaddition) of anthracene with maleic acid anhydride, using Xylene as solvent. 2. Reaction Equation O O O O O + 3. O Mechanism O O Xylene O 2 Synthesis of 9,10,11,15-tetrahydro-9,10[3’,4’]-furanoanthracen-12,14-dione 4. by Markus Arnoldini Physical properties of the substances Anthracene [1] molar weight density melting point boiling point refraction index WGK GK (CH) R-phrases S-phrases 178.24 g/mol 215 - 217 ºC 2 4 - molar weight density melting point boiling point refraction index WGK GK (CH) R-phrases 98.06 g/mol 1.32 g/cm3 (55 ºC) 51 - 53 ºC 202 ºC (1013 hPa) 1 3 R 22 (harmful if swallowed) R 34 (causes burns) R 42/43 (may cause sensitisation by inhalation and skin contact) S-phrases S 22 (do not breathe dust) S 26 (in case of contact with eyes, rinse immediately with plenty of water and seek medical advice) S 36/37/39 (wear suitable protective clothing, gloves and eye/face protection) S 45 (in case of accident or if you feel unwell, seek medical advice immediately, show the label where possible) Maleic acid anhydride [1] O O O 3 Synthesis of 9,10,11,15-tetrahydro-9,10[3’,4’]-furanoanthracen-12,14-dione by Markus Arnoldini ortho-Xylene [1] molar weight density melting point boiling point refraction index WGK GK (CH) R-phrases S-phrases 106.17 g/mol 0.88 g/cm3 (20 ºC) -25 ºC 144.4 ºC (1013 hPa) 2 4 R 10 (flammable) R 20/21 (harmful by inhalation and contact with skin) R 38 (irritates skin) S 25 (avoid contact with eyes) Calciumchloride [1] CaCl2 5. molar weight density melting point boiling point refraction index WGK GK (CH) R-phrases S-phrases 2.15 g/cm3 (20 ºC) 0.78 g/cm3 (20 ºC) 772 ºC > 1600 ºC 1 R 36 (irritates eyes) S 22 (do not breathe dust) S 24 (avoid contact with skin) Experimental accomplishment 3.25 g (= 0.02 mol) of anthracene and 1.75 g (= 0.02 mol) of maleic acid anhydride were put in a 500 ml round bottom flask. 100 ml of xylene were added and the resulting mixture brought to 100 °C and stirred for about 10 min. More xylene was added, until the entire solid was dissolved. Then the solution was refluxed for 2 h at 160 °C. It changed its color from yellow to greenish to brownish. The mixture was then kept at room temperature over night. The next day the precipitated crystals were filtered off, weighted and characterized. An amount of 3.90 g (= 0.014 mol) of crystal was received. For the calculation of the yield the molar amount of received product was divided by the molar amount of reactants used, and then multiplied by 100. This calculation yields a percentage of 70 %: 0.014mol 100% 70% 0.02mol 4 Synthesis of 9,10,11,15-tetrahydro-9,10[3’,4’]-furanoanthracen-12,14-dione 6. by Markus Arnoldini Experimental setup Calciumchloride duct Reflux-cooler Thermometer drop-flask with xylene three-neck round bottom flask LaboBib© 300 50 AN AN 1500 100 0 U/min 250 500 o C 200 7. 150 AUS AUS 1000 750 Analytical results Melting point: 261.0 °C – 262.5 °C Peaks in IR Spectrum (in cm-1): 3024: 2967: 1650-1771: C-H (aromatic) C-H (aliphatic) C=O (Keto-group) 8. Sources [1] [2] http://ch.chemdat.info/mda/ch/de/ meanings of R and S phrases from Reinhard Keese: “Grundoperationen der präparativen Organischen Chemie – Eine Einführung”, 2003, S 193-197 5 Synthesis of 9,10,11,15-tetrahydro-9,10[3’,4’]-furanoanthracen-12,14-dione 6 by Markus Arnoldini