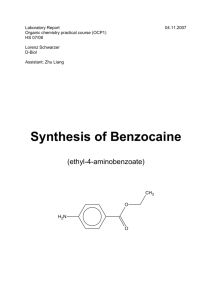
Synthesis of
advertisement

Laboratory Report Organic chemistry practical course (OCP1) HS 07/08 30.10.2007 Lorenz Schwarzer D-Biol Assistant: Zhu Liang Synthesis of Oil of wintergreen (Methyl salicylate) O O CH3 OH Method Acid-catalyzed esterification of carboxylic acid: Esterification of salicylic acid with methanol to methyl salicylate, catalyzed by sulfuric acid. Reaction equation O O O OH OH H+ + CH3 OH salicylic acid CH3 OH methanol methyl salicylate Mechanism H O O H OH O H H OH O OH H OH OH H H O H H O O O O O H O O H OH OH 2 OH Physical properties of the substances Salicylic acid O OH Molar weight Density Melting point Boiling point R-Phrases 138,12 g/mol 1,44 g/ml 158-161 °C °C R22 Harmful if swallowed, R37/38 Irritating to respiratory system and skin R41 Risk of serious damage to the eyes. S26 In case of contact with eyes rinse and seek medical advice. S39 Wear eye/face protection OH S-Phrases Methanol Molar weight Density Melting point Boiling point R-Phrases OH R11 R23 R25 S2 S7 S16 CH3 S-Phrases S24 32,04 g/mol 0.79 g/ml °C 64,4-64,8 °C Highly flammable. Toxic by inhalation. Toxic if swallowed. Keep out of the reach of children. Keep container tightly closed Keep away from sources of ignition. Avoid contact with skin. Sulfuric acid (H2SO4) HO O Molar weight Density Melting point Boiling point R-Phrases S-Phrases 98,07 g/mol 1.84 g/ml 10 °C 338 °C R35 Causes severe burns S1/2 Keep locked up and out of the reach of children S26 In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S30 Never add water to this product S45 In case of accident or if you feel unwell, seek medical advice immediately S O OH 3 Methylene chloride Molar weight Density Melting point Boiling point R-Phrases Cl H Cl R20 R40 H S-Phrases S24 84,93 1.33 40-41 Harmful by inhalation. Limited evidence of a carcinogenic effect. Avoid contact with skin. g/mol g/ml °C °C Methyl salicylate Molar weight Density Melting point Boiling point R-Phrases O O R22 R36 S24 S26 CH3 OH S-Phrases 152,14 g/mol 1,18 g/ml -8 °C 222 °C Harmful if swallowed, Irritating to eyes Avoid contact with skin. In case of contact with eyes rinse and seek medical advice. Educts Substance Salicylic acid Methanol Sulfuric acid Amount Molar amount g 4.73 ~14 9.2 mol 0.0342 ~0.437 0.0938 Volume ml ~18 5 Experimental accomplishment 1. Salicylic acid and methanol were put a 100ml round bottom flask. With heating and swirling, the salicylic acid was dissolved. Sulfuric acid was added dropwise – some white precipitate was visible. A boiling chip was added and the mixture was boiled at 60°C for 1h under reflux. 2. The solution was cooled to room temperature, transferred to a separatory funnel and 15ml methylene chloride and 10ml distilled water was added (the solution was smelling already). The lower layer (methylene chloride) was saved. This step was repeated one time. 3. The methylen chloride layer was washed with 15ml distilled water, the aqueous layer was discarded. 4. The organic layer was washed with NaHCO3. 5. The methylen chloride and the remaining water were separated by distillation (small distill apparatus). 6. The product was weighted and the refractive index and the infrared spectrum were measured. 4 Experimental setup Reflux condenser Thermometer Oilbath 67 4 5 8 3 11 9 21 4 5 3 2 1 67 8 9 1 0 Heating apparatus Separation funnel Thermometer Condenser Oilbath 67 45 8 3 11 9 21 4 5 3 2 1 67 8 9 1 0 Distillation Apparatus 5 Results Yield: Amount of salicylic acid: Amount of the product: Yield: 4.73 g 3.93 g 83 % Refractive index: Token: Methyl salicylate (lit.): 1.528 1.535-1.538 IR-Spectrum-bands [cm-1] O-H stretch (alcohols) O-H stretch (carboxylic acids) C=O stretch (esters) 3200-3500 2500-3600 1735-1750 With the aid of the comparisons of the token IR-spectrum with the reference spectrum, the product can be identified as methyl salicylate. The token refractive index confirms this result. IR-Spectrum of the Product 6 Reference IR-spectrum of methyl salicylate (red: IR of the Product) Literature http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/cre_index.cgi?lang=eng http://en.wikipedia.org http://www.discoverygate.com 7