Preliminary draft.
advertisement

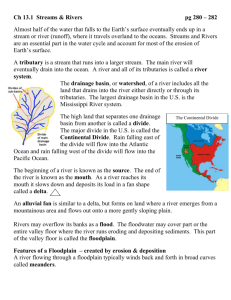
Preliminary draft. LARGE RIVERS OF SOUTH AMERICA: toward the new approach J.J. Neiff INTRODUCTION It is likely that water consumption for life maintenance is the most important of the multiple uses of water. In great part of the biosphere, water is very scanty, or it is even unattainable, and, what is even more serious, a progressively less amount of water can be devoted to direct consumption because of the increasing of pollution. Therefore, an increasing number of people counts on a less amount of water. It is just as worrying that the loss of the water quality also affects all these forms of life in the biosphere, being it now expected a decrease in the biodiversity at the continents level, with impact on human populations, an even more difficult fact to measure. Superficial waters are the most reachable for men, but only continental fresh waters allow its direct use for human, plant and pets consumption. Diagrammatically, availability of superficial water at continental level depends on the positive water balance of rains and the landscape physiography to retain it, accumulate it, or allow its runoff towards the sea. For an equal amount of superficial available water, continents have different proportion of cumulated water (bodies of lentic water) and running water (lotic environment). South America is in a privilege situation since it has a greater volume of flow of superficial running waters, that result from the constant flow starting with some rain drops and reaching the sea. This fact ensures countries possibilities to dispose of "new" and clean water in the future, under the condition that the adequate use of the basins ecosystems can prevent the erosion processes, pollution caused by the pour of toxic substances, or by the effect of acid rains. The design and adequate monitoring of the different programs of flood control in urban conglomerates, hydric exploitation for the energetic production, navigation and other possible alterations of the pulsatile regime is just as important. South American's geographic singularity: large rivers As Morello (1984) describes in his "South American ecological profile", the surfaces of the South American continent double those of Europe and reach the southern latitudes of the biosphere. However, owing to its triangular shape with it vertice looking south, its climate has a great oceanic influence, and as a consequence, it is warmer than the one of the same latitude in the northern hemisphere. Orographically, South America is an asymmetric continent, with a continuous mountain chain (the Andes Mountain Range) the height of which reaches the troposphere and two shields or high cores: the Guayana and the Brazilian one. The remaining surface corresponds to big pieces of flatland with little concave surfaces, except in the austral Andes region and in a part of the Patagonia, where there is a great number of lakes of glacial origin formed in the pleistocene that are, currently, under a tempered climate. Most runoffs in South America have sense and general W-E direction (Orinoco, Amazonas rivers) and most of the water and sediments moving along the continent are originated in the Andes mountains (of alkaline tendency, with a great amount of sediments of fine silt-sand and loam texture and a less amount of clay). A minor amount of water runs-off with a predominant North-South direction (Paraguay, Paraná and Uruguay rivers) with neutral to slightly acid waters and unselected sediments (clay to coarse sand), coming from the geological erosion of the shield of Brazil. According to the orographic origin of waters, and to biotic transformations that take place in vast flatlands, waters running along the rivers may be: "white water" (with a great amount of fine slit-sand and loam of the Andes Mountains); "black waters" (with few suspended sediments and a high content of dissolved and particulate organic matter); and "clear" (with intermediate characteristics). This simple classification developed by Sioli (1975) for the basin of the Amazonas river more than 30 years ago is currently applicable today to most large rivers in the continent. Based on Troll's criteria to the north of Buenos Aires, the warm and wet maritime climates prevail (OAS, 1973). They receive their humidity and rains from the atmospheric circulation in the Atlantic ocean. Resulting from this physiographic and climatic characteristics, South America is the continent where large rivers collect the greatest amount of superficial water to spill it into the Atlantic ocean. The three largest basins of the continent (Orinoco, Amazonas and Paraná), contribute with 13% of the total suspended solids delivered by all rivers to the oceans (Tundisi, 1994). In the table 1 made with data provided by Welcomme (1985) and Neiff et al., (1994), is possible to see the importance of large rivers in South America compared to other continents. Discharge (table 1, column 1), in relation to the surface of each basin, is always greater in South America than in other continents (see: rate 2/1 in the table). When comparing the surface of continents, and the discharge of large rivers, it results that the amount of running water in relation to the continental surface is much greater in South America (Fig. 1). Brief comparison of lakes and large rivers functioning in South America In most South American lakes, superficial waters are of Pleistocene origin and have accumulated disturbances from geologic times, and the impact of antropic activities (control of water in tributary rivers, contaminants, sediments). In large South American river waters, only stays for some months flowing along the continent, thus carrying and transferring minerals and organisms through the basin. Let's us exemplify, in lakes the percentage of the volume of water annually renewed is very low compared to the volume comprised in the basins. Water circulation is produced in one or more seasonal periods, and the mixture efficacy essentially depends on the physical attributes, specially in temperature. The periodic circulation of water depends greatly on the amount of solar energy that the mass of water receives locally. Lakes, therefore, can be considered as systems with great potential energy and low kinetic energy. From the energetic point of view, they can be considered as "cummulators" with a slow active volume: the hypolimnion. In South America, large rivers can comprise small and large lakes in their basin, as well as wetlands, other lentic environments, but the greatest volume of water is temporarily or permanently in horizontal movement. The water renewal rate, in a determined section, is high compared to cumulated water. The concentration of elements (nutrients, organisms, sediments) must be expressed in relation to the discharge values and not in volume units. The greatest amount of power that goes through the system is kinetic and this is of great importance when analyzing the nutrients flow, temporal distribution patterns of organisms, the use and handling of rivers. Physiographic differences and, particularly the land slope, can determine the presence of tributaries and quick runoffs and slow runoffs sectors. These latter do not have the typical characteristic of vectorial flow (typical of rivers) since they can run-off in one or other direction during the different periods of the year, depending on the volume of flow of the collector stream. The table 2 shows some characteristics that enable to compare rivers with mountain vectorial landscapes to rivers with flatland landscape (equipotential) from the concept submitted by Gonzalez Bernaldez (1981). Consequences of the movement of water in large rivers The main difference as regards lakes is the horizontal movement of water and, besides, between small and large rivers is that in the former case, water moves only temporarily while in large rivers, flow is permanent and organizes the distribution patterns and the organisms abundance. They are, therefore, systems or macrosystems where water, nutrients, sediments and organisms pass through a certain runoff section at a certain speed. For a better understanding of the functioning of the fluvial macrosystem variability, a "typical" lake (for example the Mascardi lake, Rio Negro, Argentina) can be compared to a dam lake (for example, Yaciretá dam on Paraná River at Argentina). A very simple example made up with a glass (with the lake volume, relatively constant) and two tubes: one is the income of water and the other is the outlet of superficial water, can be applied. 2 Volume (v) comprised in the glass is the cumulated information (generally speaking) in a certain time (t). If water were not renewed (utopia) the internal organization would depend on the amount and quality of elements comprised in the glass (nutrient species, etc.), on the energy fluctuations that our "glass" (or water body) seasonally receives and on interactions of elements within the system. In lakes: Q1 S Q2 Total internal change: TTRi P E S Q1 Q2 where: P = Energy inflow (precipitation, solar energy) E = Energy outflow (runoff, termal advenction, etc.) S = Surface area Q1 = Inflow of information (water, sediments, spp.) Q2 = Outflow of information (water, sediments, spp.) t = time But in rivers: Q1 V Then: Total turnover rate Q2 TTR 1 Qt12 / V TTRi Total turnover time TTt = 1/TTR We would now place the income of water tube (nutrients, sediments, organisms) Q 1 in the graphic, and the outlet tube that will bear the Q2 symbol. In this second example there is, besides the internal metabolism of the system, an inflow of information (nutrients, sediments, organisms) unit of time. Normally, in lakes and rivers, the volume is relatively constant and the income and outlet volume of flow vary in an analogous way. The turnover rate (TTR) is the percentage of the total water comprised in the glass that comes in or out in a certain period of time. The turnover time is reciprocal to the turnover rate and states the necessary time for a complete renewal of water in the glass. If the glass has a 1 litre capacity and 100 ml income per day, the turnover rate will be 100/1000, or 0,1 or 10 per cent per day. Both rates are of significant use in order to value the exchange of information of the system under analysis. In practice, the turnover time rate is generally used. The TTR would be different along the river sections. Values of nutrient concentration in rivers offer a small amount of information if not discussed with the volume of flow data crossing this point or section. Turnover time for the Yacyretá dam (placed in the watercourse of the Alto Paraná) is about 3 weeks. This same assessment, made for the Mascardi lake, has an approximate value of five years. Water renewal in large rivers (and of other elements of the system) is quite high as regards the information volume comprised in the system. For such reason, the indices application described in the system status cannot be the same to those used in the study of systems of low turnover (as it happens in most lakes). The biocenosis analysis with the use of indices of dominance, abundance, equitability, diversity and other, of known use in low turnover time ecosystems (Hulbert, 1971), have a low use to indicate the organization complexity and the functioning of communities that live in large rivers. Most known indices 3 express the organisms distribution in a number of species. The disadvantage that they have is that they do not incorporate the turnover-time and turnover-rate magnitude. Going back to our example of the glass: an increase of 10 individuals (or species or information units, generally speaking) can give the same result even when the flow rate in the system varied in great manner. If the outlet rate (death, emigration) were 0, the change rate would be 10. But it would also be 10 if 200 individuals were incorporated, with a 190 exit, or if 1000 were incorporated and 990 got out. These indices are not very sensitive to explain the changes in systems with a high populational turnover due to the horizontal movement of the water during floods in the river valleys. They can also end up in misleading conclusions since in several occasions the diversity is kept with small change even when there is a 60% renewal of the species forming the community between high waters and low waters phase (Frutos, 1993; Zalocar de Domitrovic, 1993). Still when comparing extreme situations of low waters and others with extraordinary flood, the specific diversity does not reflect significant contrasts. The use of the simplest similarity index (as the one of Sörensen) to different situations of extreme high and low waters state that similarity is less to 30% for phytoplankton (Fig. 2). In biotic systems of rivers, mainly in those of high change rate as planktonic groups or those of invertebrates that live in plants the complexity analysis requires to know the change rate, the response time and the possibility for a population or community to repeat its structure throughout time (Poi de Neiff and Bruquetas, 1989; Huszar, 1994). In order to graphically represent this idea: perception is completely different when we observe the blades of a fan which is not working (that is to say, without performing its essential function) and the one we obtain when the blades move at different revolutions per minute. In our example of the fan, we should now think that each blade is a species (or population or bioform) and that our "fan" could have "X" number of blades (as many as different elements that form our community) each of them represented in a different color. Therefore, our perception "would have a different color" by the number of colors (species, elements) forming the blade, and the speed we give to the fan. Yet, the problem gets more complex since in the case of rivers, changes are not produced in the form of cycles (bio-geo-chemical cycles are not cyclic within the system) and because flows are produced as energy pulses and matter pulses presenting flood phases and dry phases. Both phases form the "hydro-sedimentologic pulse" (or simply "pulse"). And the way of these pulses is variable throughout the century, and even in a decade. Variability throughout the long series of time shows regularities that may be profitably studied by the use of the rescaled range analysis since it allows to find hydrologic variability tendencies (Armengol et al., 1991). In rivers, the amount of water that goes through a certain section and in a certain unit of time varies generally in a sinusoidal way, as a consequence of the rain distribution, physiography and basin soils. Frequency, scale and duration of dry and flood phases depend on the topographic position of the river islands and on the ecosystems occupying the floodplain, and they will also receive -with a greater or less frequency- the hydrologic events that take place during summer or in winter. Each river, or section of the river receives with a greater or less regularity a flood phase or a dry phase of certain magnitude. This group of hydrologic attributes defines the characteristics of the pulsatile system or "FITRAS" function (Frequency, Intensity, Tension, Regularity, Amplitude and Seasonality) as they were defined in previous contributions (Neiff, 1990b, Neiff et al. 1994). In the figures 3 and 4 and the FITRAS function is graphically described, thus being different according to the different topographic levels of the floodplain. Ecologic consequences of the pulse system It is generally known that the floodplain landscape of large South American rivers is very different from the high land ecosystems limiting the river. It is also known that there are biotic differences among the different sectors of the watercourse and of the floodplain. In large rivers with floodplain laterally located (fringe-floodplains, in sensu Wellcomme, 1985), it is possible to find a growing complexity as regards organization in a transversal sense of that of the watercourse of the river. Marchese & Ezcurra de Drago (1992) describe a typical zonation with complexity increase (amount of species, specific diversity, trophic niches) from the main watercourse to the minor tributaries (Fig. 5). This increase in the richness of species in a section transversal to the Low Paraná was related with modifications in 4 the physical and chemical properties of the environment (discharge, sediments texture, organic substance, dissolved oxygen). For phytoplankton and zooplankton (Zalocar 1990, 1992, 1993) the trends are similar (table 3). Junk et al. (1989) explained that a significant part of the biotic organization of rivers with floodplain is ruled by "flood pulses" and that periodic flood events produce stress situations for biota, reflected in a system "resetting". Bonetto (1975, 1976) explained that floods produce "rejuvenation" processes in ecosystems forming part of the river. Biocenosis of large rivers are regulated by the hydrodynamic of pulses. But low water phases are as important as flood periods (Neiff, 1990b; Neiff et al. 1994). And this is not only a semantic problem regarding the "flood pulse" concept given by Junk et al. (op. cit.). During this dry phase, plants suffer from an acute stress, as it has been documented (Neiff and Poi de Neiff 1990) due to the leaves abscision and growth arrest. Vertebrates fauna find a less amount and quality of environments during this dry phase, therefore a concentration (overload of individuals) is produced in a surface up to 5 to 10 times less than the one of the flood period, and animals are more vulnerable. I desire to emphasize that the dry phase is a powerful selection factor on the distribution and abundance of the animals and plants. Most of the fish populations can not survive, or suffer important losses during very extended droughts. (Merron et al., 1993). Floods represent a change macrofactor in the biotic structure. However, there are trees with morphologic, anatomic and physiologic adaptations to perform their photosynthesis even under immersion conditions (Joly and Crawford, 1982; Fernandes Correa and Furch, 1992; Neiff and Reboratti, 1989; Tundisi, 1994). Some trees stay up to nine months with the soil covered by water without noticeable modifications in their growth, even in cases of long-term floods that cause the death of a great amount of trees of the gallery forest in one year (Neiff et al. 1985). The phenology of some species of the Amazon varzeas would not be affected by the floods (Oliveira, 1995). Rooted vegetation of floating leaves growing in the floodplain lakes has ecophenes of its own, of the flood phase and the dry phase (Junk 1970; Neiff, 1979). When covered by flood water, they speed up their growth and modify their anatomy and morphology (Neiff, 1979), thus attaining -during this stress phaseproductivity values up to five times greater than the average annual productivity value (Neiff 1990a) as it is shown in table 4. This productivity response has not been noticed during the flood phase in the Amazonas floodplain (Junk, 1986). Some tools to get to know the biotic variability of large rivers The water temperature regime of the South America large rivers is little known and requires highpriority as a tool within the Global Change Program. Interactions among physical, chemical and biological factors at regional and local level need be incorporated in the thermal study of these rivers (Budiko et al., 1994). Species richness in a determined environment or section of a river, the use of productivity or the amount of individuals can help as indicators of the system's complexity (Fig. 5). It is quite useful to analyze the biotic variability in pulsatile systems, employing rates that combine populations abundance according to ranges, with respect to frequency values in the community (McNaughton & Wolf, 1984). The shape of these curves provides information as regards stress situations that take place during long floods (Fig. 6) in different biotic groups (Poi de Neiff and Bruquetas 1989). During the critical periods the diversity decrease and the distribution of the relative abundance shows a geometric curve. We could be use an index that combine three biotic parameters: abundance, as mean density (or individual numbers in each hydrologic phase, or better: moment within the phase); frequency, i.e. the number of phases (or moments within the phase) occupied, as expression of the niche amplitude; and the mean weighted or barycentre, i.e. the mean weighted by the density of population in each hydrologic phase, to evaluate the position of a population or populations for a given hidrologic curve. Finally: 5 h Nh X h PPh h Nh where: PPh = Population position in a hydrosedimentologic pulse. X h = Mean coordinate weighted by density of population in each pulse h. Nh = Population density in the hydrologic phase (or date) h. The "ABC method" (abundance/biomass comparison) were proposed by Warwick (1986) for detecting pollution effects on macrobenthic communities. Thereinafter, Meire and Deren (1990) proposed the ABC index: ABC Bi A i N where: Bi = % dominance of species i (ranked from the highest to the lowest biomass) Bi = % dominance of species i (ranked from the most to the least abundant species) N = total number of species. The index is negative in heavily stressed conditions and positive in unstressed conditions. The number of times the cumulative percentage dominance as the percentage of the total number of species minus one (cumulative biomass dominance). Coeck et al. (1993) concluded that the method gives information about both pollution and physical disturbance. I think that ABC index can be tested to analyze the fluctuations of the communities induced by the river regime (pulses). For certain studies, it is necessary to know which population/s is/are the one/s which constantly occupies/y an important space or volume within the system throughout a series of time, in relation to other biotic components of the system. For such purpose, I suggest the use of the prevalence index: Prevalence index: P 2 Ui / N t o ... t1 n spp 0 where: Ui = Unit of importance (Productivity, Density, Other) N = Importance value magnitude This index can be used in an attempt to explain the dynamics of groups in ecosystems regulated by pulses, and specially, in those with a high change rate, due to marginal flows (in Lewis et al. concept 1990) such as plankton of floodplain lakes that exchange the water with the one of the river course. Such as other indices, it can be of use to analyze the biotic variability in long series of time, since it gives place to an idea of prevalence and persistence of populations in environments with a great variability. One of the generally accepted ideas is that the biotic complexity, mainly the species richness, increases towards the lower sector, towards the mouth. This does not occur in rivers such as Paraguay-Parana (Neiff, 1990b) where the greater biotic complexity is found in the Gran Pantanal of Matto Grosso, in the high basin of Paraguay. This complexity is related to the extension and spatial variability of wetlands comprised in the basins of these large rivers, and with the FITRAS hydrologic function along the river (Neiff, op.cit.). But, also the temperature fluctuations are one of the most important causes of the biotic complexity in tropical rivers. Production of organic matter 6 In Patagonian lakes of South America, greater productivity is generally concentrated in the top of the water column, with low values in the hypolimnion. The greatest part of the trophic nets are based on the phytoplankton. Magnitudes can vary in different lakes, but the productivity gradients are vertical. In large rivers of the South America, productivity is generally greater when considering the basin as an analysis unit. The greatest amount of energy is captured by macrophytes and by floodplain forests (Neiff, 1990a). Efficiency in the accumulation of energy has differences in transversal transections to the watercourse, with higher values towards the floodplain. Assessments for the potamophytoplankton are 3-6 tn.ha.year-1 (García de Emiliani y Anselmi de Manavela, 1983; Perotti de Jorda, 1980; Rai and Hill, 1984), while vegetated lakes with Eichhornia crassipes attain values from 12 to 16 tn.ha.year-1 (Perez del Viso et al., 1968; Lallana, 1980; Neiff and Poi de Neiff, 1984; Junk, 1986), and the gallery forest of the islands of some rivers have frequent values of 20-36 tn.ha.year-1 (Neiff, op.cit.; Neiff and Reboratti, 1989). Utilization of phytomass and necromass Currently, the percentage of this primary production transferred to consummers is not known and, what is even less known is the efficiency throughout trophic nets. The direct consumption of plants is low, on the one part due to the low amount of herbivorous and to a great amount of plants with hard tissues that turn out to be almost trophically unattainable. A great part of the incoming power through productivity of the plants is captured by consummers from the organic detritus in different processing grade. This is not exclusive of large South American rivers (Vannote et al., 1980; Cummins et al., 1983). The main difference is that in these South American rivers with large flood plains, the organic debris do not come from the same earthly ecosystems, but from the vegetation growing in the same river, and therefore, it is autochthonous, as in the detritus originated therein. The organic matter produced is always high, mainly owing to the vegetation contribution of wetlands comprised in the floodplain. Respiration is low since the oxygen in the water is quickly exhausted, giving place to the formation of intermediate organic components that characterize "clear waters" and "black waters", and to an organic stock that remains in the system as necromass. There is a lot of bibliography stating that rivers behave as "heterotrophic" systems since respiration is higher than production. In large South American rivers, great rate variations take place: P/R when considering different subbasins. However, rivers with large flood plains have values higher to 1, considering the river as it is: the watercourse + its floodable plain. It will be convenient to avoid the use of terms originated in the Limnology of lakes to define the trophic condition of rivers, since the P/R balance is conditioned by the horizontal movement and the permanence time of the water in the sector under analysis. For such reason, the capacity of the system is not expressive to capture the energy from nutrients and transmit it through the trophic nets. The P/R rate in these large rivers is also very difficult to estimate, since the income of nutrients depends greatly on the high basin and the lateral flows in the floodable plain (from and to the watercourse). To get a quick idea of the basin metabolism (or a sector of it), it would be of great use to know the quantity and quality of the organic substance transmitted, and the saturation level of oxygen in the water. Both represent the "fuel" and the "combustive agent" from the capture and accumulation of power to the disintegration of organic substance (Neiff, 1990b). Both magnitudes attain characteristic values for each hydrologic phase of such basin. The organic stock magnitude for two flood phases with the same magnitude, also depends on the amplitude duration of the previous dry phase and the moment of the year when flood takes place. The concentration of organic carbon and its fractions, permit a sintetic idea of the metabolism of the river basin. A comparison between Uruguay and Paraná suggest a more significant role of the latter as a biodegrading water body: carbon in particulate aminoacids and sugars seems twice is abundant in the Uruguay (Mañosa and Depetris, 1993). According to Richey et al. (1991) DOC represents 50% of the total carbon transported by Amazonas and the dissolved humic compounds represents 60% of the DOC. The size of particles of the organic substance present in large South American rivers is of significant importance as a synthetic indicator of processes that occur in the basin (Mulholland, 1981; Degens and Ittekkot, 1985). When the slope and the water volume of flow are similar, the percentage differences among 7 the thick organic substance content, fine particulated and dissolved, depends on the oxygen availability in the water; and this latter depends greatly on the flow conditions (volume of flow, residence time of the water). The amount of total organic carbon and the proportions of POC and DOC in the waters depend of the hydrologic phase of the pulse with a higher values during the extraordinary floods (Kempe and Depetris, 1990; Depetris and Kempe, 1993). A selection process favouring the use of the organic debris has been produced, since it is a trophic resource always present. According to Bonetto (1976) 60% of fish productivity of the Parana river is concentrated in detritivourous fish. Works by Bowen et. al. (1988) have stated important morphologic, physiologic and selective food behavior adaptations of detritivorous fish. Decomposition of organic substance The decomposition rate of the organic matter expressed by the "k" coefficient (Olson, 1963) allows the exploitation of the P/R rate for different ecosystems of the basin of these large rivers; investigating the efficiency of the decomposition process during the flood-phase (potamophase) and the dry-phase (limnophase). It also allows the comparison of the kinetic disintegration of the organic matter for species growing from semi-lotic waters to the external border of the floodplain. The available information for the Paraná basin (Poi de Neiff and Neiff, 1988,1989; Hammerly et al. 1989; Poi de Neiff y Bruquetas, 1991; Bruquetas and Neiff, 1991; Neiff and Poi de Neiff, 1990; Poi de Neiff , 1991; Bruquetas de Zozaya and Poi de Neiff, 1993) allows to summarize some tendencies. The decomposition rate mainly depends on two principal factors: quality of organic substance (lignin: nitrogen ratio or better C:N ratio), and the oxygen availability in the water. The duration and the dry-phase magnitude have consequences over the process, and the moment of the year in which it takes place is just as important. The time required for a 95% loss of Tessaria integrifolia, Salix humboldtiana, Polygonum accuminatum, Panicum grumosum and Eichhornia crassipes leaves are 88, 158, 176, 353 and 50 days respectively, under aerobic conditions. The decomposition rate of E. crassipes leaves is 3 times quicker in well oxygenated waters of connected lakes during the greatest part of the year to the watercourse of the river that in anaerobic conditions of lakes sporadically coupled to the river. For a 95% decomposition of P. repens, Thalia multiflora and Typha latifolia leaves the estimate time were 325, 545 and 1000 days respectively, under anaerobic conditions. When the water level lowers abruptly and the soil remains dry, the decomposition time of the necromass of the same plant doubles (Bruquetas and Neiff, 1991). During winter, the necromass that breaks up in the water finds a 15-18ºC temperature while during summer, the process occurs between 22 and 32ºC. Finally, the production of organic matter (Neiff, 1990a) is equal to, or higher than, the one broken up (Poi de Neiff, 1991 and 1993) in a river with floodplain as the Low Paraná. The P/R rate is closely related to the pulse function (FITRAS). The hydrosedimentologic regulation of the river, and specially the additive effects of dams can alter the FITRAS function in some or all the ecosystems of flood plains. From this alteration, changes in the P/R quotient, and in the quality and quantity of organic debris available for consummers can be expected. The chain effects deserve special investigations. 8 References ARMENGOL, J., SABATER, S., VIDAL , A. AND SABATER, F., 1991. Using the rescaled range analysis for the study of hydrological records: the river Ter as an example. Oecologia Aquatica 10: 21-33. BONETTO, A.A., 1975. Hydrologic regime of the Paraná river and its influence on ecosystem. In: HASSLER, A. (ed.): Coupling of land and water systems. Springer-Verlag, New York: 175-198. BONETTO, A.A., 1976. Calidad de las aguas del río Paraná. Introducción a su estudio ecológico. Dir. Nac. Constr. Port. y Vías Navegables. INCYTH-PNUD-ONU. 202 p. Buenos Aires. BOWEN, S., AHLGREEN, M.O. and NEIFF, J.J., 1988. Diet selection by an exceptionally detritivorous fish, Prochilodus platensis, in the Rio de la Plata System. Annual Meeting of the Amer. Fish. Soc. Toronto. BRUQUETAS, I.Y. and NEIFF, J.J., 1991. Decomposition and colonization by invertebrates of Typha latifolia L. litter in Chaco cattail swamp (Argentina). Aquatic Botany 40: 185-193. BRUQUETAS de ZOZAYA, I.Y. and POI de NEIFF, A.S.G., 1993. Descomposición de macrófitos en bañados de la planicie inundable del río Paraná. Ambiente Subtropical 3: 1-17. BUDIKO, M.I., BORZENKOVA, I.I., MENZHULIN, G.V. and SHIKOMANOV, L.A., 1994. Cambios antropogénicos del clima en América del Sur. Publ. Acad. Nac. Agron. y Veterinaria. Buenos Aires, Argentina. 223 p. CARIGNAN, R. and NEIFF, J.J., 1992. Nutrient dynamics in the floodplain ponds of the Paraná River (Argentina) dominated by Eichhornia crassipes. Biogeochemestry 17: 85-121. COECK, J., VANDELANNOOTE, A., YSEBOODT, R. and VERHEYEN, R.F., 1993. Use of the abundance/biomass method for comparison of fish communities in regulated and unregulated lowland rivers in Belgium. Regulated Rivers: Research & Management, Vol. 8: 73-82. CUSHING, C.E., MC INTIRE, C.D., CUMMINS, K.W., MINSHALLL, G.W., PETERSEN, R.C., SEDELL, J.R. and VANNOTE, R.L., 1983. Relationships among chemical, phisical and biological indices along river continuo based on multivariate analysis. Arch. Hydrobiol. 98: 317-326. DEGENS, E.T. and ITTEKKOT, V., 1985. Particulate organic carbon. An overview. In: DEGENS, E.T., KEMPE, S. and HERRERA, R. (eds.): Transport of Carbon and Minerals in Major World Rivers, Pt.3. Mitt. Geol. Palaont. Inst. Univ. Hamburg, SCOPE/UNEP Sonderbd 58: 7-27. DEPETRIS, P.J. and KEMPE, S., 1993. Carbon dynamics and sources in the Paraná River. Limnol.and Oceanogr. 38(2): 382-395. DRAGO, E.C., 1994. The physical limnology of the river-lake systems of the Paraná River floodplain. In: Sustaining the Ecological Integrity of Large Floodplain Rivers. Internat. Conference, U.S. Dep. of Interior, Nat. Biol. Survey, Univ. of Wisconsin. La Crosse Wl, July 12-15, 1994. (In press). FERNANDEZ CORREA, A.F. and FURCH, B., 1992. Investigations on the tolerance of several trees to submergence in blackwater (Igapó) and whitewater (Varzea) inundation forests near Manaus, Central Amazonia. Amazoniana XII (1): 71-84 FRUTOS, S.M., 1993. Zooplancton en cuerpos de agua isleños del Bajo Paraná. Ambiente Subtropical 3: 87121. GARCIA de EMILIANI, M.O. y ANSELMI de MANAVELA, M.I., 1983. Fitoplancton de los principales cauces y tributarios del valle aluvial del río Paraná: tramo Goya-Diamante, II. Rev. Asoc. Cien. Nat. Litoral 14(2): 217-237. GONZALEZ BERNALDEZ, F., 1981. Ecología y Paisaje. Ed. Blume, Madrid. 275 p. 9 HAMMERLY, J.A., LEGUIZAMON, M., MAINE, M.A., SCHIVER, D. and PIZARRO, M.J., 1989. Decomposition rate of plant material in the Paraná medio (Argentina). Hydrobiologia 89: 53-59. HULBERT, S.H., 1971. The neoconcept of species diversity: a critique and alternative parameters. Ecology 4: 577-586. HUSZAR, V.L. de MORAES, 1994. Fitoplancton de um lago amazónico impactado por rejeito de bauxita (lago Batata, Pará, Brasil): estructura da comunidade, fluctuaçoes espaciais e temporais. Doctoral Tesis, Univ. of Sao Carlos - SP, Brasil. 219 p. IRMLER, U., 1982. Litterfall and nitrogen turnover in an Amazonian inundation forest. Plant and Soil 67: 355358. JOLY, C.A. and CRAWFORD, R.M.M., 1982. Variation in the tolerance and metabolic response to flooding in some tropical trees. J. Exp. Bot. 33: 799-809. JUNK, W.J., 1970. Investigations on the ecology and production biology of the "floating meadows" (paspaloEchinochloetum) on the Middle Amazon. l. The floating vegetation and its ecology. Amazoniana 2: 449-495. JUNK, W.J., 1986. Aquatic plants of the Amazon system. Pp. 319-337. En: WALKER, K.F. and DAVIES, B.R. (eds.): The Ecology of River Systems. Dr Junk Publ. The Netherlands. JUNK, W.J., BAILEY, P.B. and SPARKS, R.E., 1989. The flood pulse concept in river-floodplain systems. Pp. 110-127. In: DODGE, D.P. (ed.): Proc. of the Internat. Large River. Symp. Can. Spec. Pbl. Fish. Aquat. Sci. 106. KEMPE, S. and DEPETRIS, P.J., 1990. Labile carbon in the Paraná river basin. Mitt. Geol. Palaont. Ins. Univ. Hamburg 69: 157-165. LALLANA, V., 1980. Productividad de Eichhornia crassipes (Mart.) Solms en una laguna isleña de la cuenca del río Paraná Medio. II: biomasa y dinámica de población. Ecología 5: 1-16 (Argentina). LEWIS, W.M., WEIBEZHAN, F.H., SAUNDERS III, J.L. and HAMILTON, S., 1990. The Orinoco river as an ecological system. Interciencia 15(6): 346-357. MAÑOSA, W. and DEPETRIS, P.J., 1993. Preliminary results on carbon fluxes in the Uruguay river. Mitt. Geol. Palaont. Ins. Univ. Hamburg 74: 13-22. MARCHESE, M. and EZCURRA de DRAGO, I., 1992. Benthos of the lotic environments in the Middle Paraná River System: transverse zonation. Hydrobiología 237: 1-13. MCNAUGHTON, S.J. and WOLF, L.L., 1984. Ecología General. Ed. Omega, Barcelona. 388 p. MEIRE, P.M. and DEREU, J., 1990. Use of the abundance/biomass comparison method for detecting environmental stress: some considerations based on intertidal macrozoobenthos and bird communities. J. Appl. Ecol., 27: 210-223. MERRON, G., BRUTON, M. and LA HAUSSE de LALOUVIERE, P., 1993. Changes in fish communities of the Phongolo floodplain, Zululand (S. Africa) before, durinig and after a severe drought. Regulated Rivers 8: 335-344. MORELLO, J.H., 1984. Perfil Ecologico de Sudamérica. ICI (Instituto de Cooperación Iberoamericana). Barcelona. 93 p. MULHOLLAND, J., 1981. Deposition of riverborne organic carbon in floodplain wetlands and deltas. In: Carbon Dioxide effects Research and assessment flux of organic carbon by rivers to ocean. CONF8009140, U.S. Dept.of Energy, Washington DC: 142-172. NEIFF, J.J., 1978. Fluctuaciones de la vegetación acuática en ambientes del valle de inundación del Paraná Medio. Physis (B) 38: 41-53. 10 NEIFF, J.J., 1990a. Aspects of primary productivity in the lower Paraná and Paraguay riverine system. Acta Limnol. Brasil. III: 77-113. NEIFF, J.J., 1990b. Ideas para la interpretación ecológica del Paraná. Interciencia 15 (6): 424-441. NEIFF, J.J., IRIONDO, M.H. and CARIGNAN, R., 1994. Large tropical south american wetlands: an overview. Pp. 156-165. In: LINK, G.L. and NAIMAN, R.J. (eds.): The Ecology and Management of Aquaticterrestrial Ecotones. Proccedings book, Univ. of Washington. 225 p. NEIFF, J.J. y BRUQUETAS de ZOZAYA, I.Y., 1991. Decomposition and colonization by invertebrates of typha latifolia L. litter in Chaco cattail swamp (Argentina). Aquatic Botany 40: 185-193 NEIFF, J.J. y POI de NEIFF, A., 1984. Cambios estacionales en la biomasa de Eichhornia crassipes y su fauna en una laguna del Chaco (Argentina). Ecosur 11(21/22): 51-60 NEIFF, J.J. y POI de NEIFF, A., 1990. Litterfall, leaf decomposition and litter colonization of Tessaria integrifolia in the Paraná River floodplain. Hydrobiologia 203(1-2): 45-52. NEIFF, J.J. y REBORATTI, H.J., 1989. Estructura y dinámica de bosques de Tessaria integrifolia. II. Análisis de crecimiento y productividad. Bol. Soc. Arg. Bot. 26(1-2): 39-43. NEIFF, J.J., REBORATTI, H.J., GORLERI, M.C. y BASUALDO, M., 1985. Impacto de las crecientes extraordinarias sobre los bosques fluviales del Bajo Paraguay. Bol. Com. Espec. Río Bermejo. Cámara de Diputados de la Nación (Buenos Aires) 4: 13-30. OLIVEIRA, C., 1995. Phenological studies of Salix humboldtiana in flooded forest (varzea) in Central Amazonia. Book of Abstracts XXVI Congr. of SIL. Sao Paulo (Brasil), 23-29 Jul. 279 p. OLSON, J.S., 1963. Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44: 322-331. PEREZ del VISO, R., TUR, N. y MANTOVANI, V., 1969. Estimación de la biomasa de hidrófitos en cuencas isleñas del Paraná medio. Physis 49 :219-226 PEROTTI de JORDA, N.M., 1980. Campaña limnologica "Keratella I" en el río Paraná Medio: pigmentos y productividad primaria en ambientes lóticos. Ecología 4: 55-61. POI de NEIFF, A.S.G. y BRUQUETAS de ZOZAYA, I.Y., 1989. Efecto de las crecidas sobre las poblaciones de invertebrados que habitan macrófitas emergentes en las islas del río Paraná. Rev. Hydrobiol. Trop. 22(1): 13-20. POI de NEIFF, A.S.G. y BRUQUETAS de ZOZAYA, I.Y., 1991. Descomposición y colonización del detrito de distintas especies de plantas en ambientes inundables del río Paraná. Biología Acuática 15, Parte II. Notas Científicas RAL'91: 158-159. POI de NEIFF, A. y NEIFF, J.J., 1988. Decomposition of Eichhornia crassipes (Mart.) Solms in a pond of Paraná river valley and colonization by invertebrates. Trop. Ecol. 29(2): 79-85. POI de NEIFF, A. y NEIFF, J.J., 1989. Dry weight loss and colonization by invertebrates of Eichhornia crassipes litter under aerobic conditions. Tropical Ecology 30(2): 1-12. RAI, H. and HILL, G., 1984. Primary production in the Amazonian aquatic Ecosystem. In: SIOLI, H. (ed.): The Amazon. The Hague. Dr.Junk Publ. RICHEY, J.E., EDGEN, J., DEVOL, A.H., KUAY, B.D., VICTORIA, R. and MARTINELLI, L., 1990. Biogeochemistry of carbon in the Amazon river. Pp. 252-371. In: Degens, E.T., Trempe, S. and Richey, E.J. (eds.): Biogeochemistry of the major world rivers. John Wiley & Sons. New York. SIOLI, H., 1975a. Tropical rivers as expressions for their terrestrial environments. Pp. 275-288. In: GOLLEY, F.B. y MEDINA, E. (eds.): Tropical Ecological Systems. Trends in terrestrial and aquatic research. Springer-Verlag, New York. 11 TUNDISI, J.G., 1994. Tropical South America: present and perspectives. Pp. 353-424. In: MARGALEF, R. (ed.): Limnology now: a paradigm of planetary problems. Elsevier, Amsterdam. VANNOTE, R.L., MINSHALL, G.W., CUMMINS, K.W., SEDELL, J.R., and CUSHING, C.E., 1980. The river continuum concept. Can. J. Fisheries and Aquat. Sci. 37: 130-137. WARWICK, R.M., 1986. A new method for detecting pollution effects on marine macrobenthic communities. Mar. Biol., 92: 557-562. WELCOMME, R.H., 1985. River fisheries. FAO Fish. Tech. Paper 262. Rome, 330 p. ZALOCAR de DOMITROVIC, Y., 1990. Efecto de las fluctuaciones del nivel hidrométrico sobre el fitoplancton en tres lagunas isleñas en el área de la confluencia de los ríos Paraná y Paraguay. Ecosur 16 (27): 1-23. ZALOCAR de DOMITROVIC, Y., 1992. Fitoplancton de ambientes inundables del río Paraná (Argentina). Revue d'Hydrobiologie Tropicale 25(3): 175-186. ZALOCAR de DOMITROVIC, Y., 1993. Fitoplancton de una laguna vegetada por Eichhornia crassipes en el valle de inundación del río Paraná (Argentina). Ambiente Subtropical 3: 39-67. 12 River Table 1: LARGE RIVERS OF THE WORLD Annual mean discharge near the mouth (after Welcomme, 1985; Neiff et al., 1994) 1 = mean discharge (1000 m3.sec-1); 2 = drainage area (1000 km2); 3 = length (km) South America Africa River 1 2 3 1 2 Amazon Orinoco Paraná Tocantins Magdalena Uruguay Sao Francisco Total rate 2/1=40.3 212.5 17 16 10.2 7.5 3.9 2.8 269.9 5711 870 2278 896 238 230 665 10888 6437 2151 3998 2700 1600 1612 2900 21398 Congo Zambezi Niger Nile Senegal Total rate 2/1=169.8 39.7 7.1 6.1 2.8 0.9 56.7 North America Missouri St. Lawrence Mackenzie Columbia Yukon Frazer Nelson Móbile/Tombigbee Susquehanna Total rate 2/1=155.1 17.3 14.1 7.9 7.3 5.1 3.2 2.3 1.6 1.1 59.9 3184 1274 1784 660 921 235 1059 107 71 9295 Total rate 2/1=112.5 6.2 4.1 3.5 2.6 2.2 1.7 1.7 1.4 1.1 24.5 806 322 355 279 143 496 94 69 194 2758 3968 1280 1100 2944 338 9630 4700 3500 4200 6650 1633 20683 Asia 6020 4000 4241 1954 2654 1360 2570 598 710 24107 Europe Danube Pechora Dvina Neva Rhine Dneper Rhone Po Vístula 3 2850 1809 726 1312 2200 816 648 1084 11445 Yangtze Brahmaputra Ganges Yenisei Lena Irrawaddy Ob Mekong Amur Indus Kolyma Sankai 21.8 19.8 18.7 17.4 15.5 13.5 12.5 11 11 5.6 3.8 3.6 1920 924 1047 2560 2396 424 2455 793 1822 916 637 117 5980 2900 2506 5540 4400 2100 5410 4000 4444 2900 2513 1957 Godavari 3.6 294 1440 3.3 2.5 2.0 1.8 1.5 1.4 1 171.3 665 189 304 355 276 535 243 18872 4845 1056 1120 1725 2400 1900 1067 60203 Hwang-Ho Pyasina Krishna Indigirka Salween Tigris-Euphrates Yana Total rate 2/1=110.1 Table 2:Breaf comparison of flatland and mountain rivers of South America Characteristic Vectoriality Subtropical flatland rivers Minimum (equipotentials) Mountain rivers with temperate climate Vectorials Physiography Complete basin is reception-discharge Zone of head-waters - transport - delta Time of water concentration High Low Kinetic energy Low High Hydrometric regime Bimodal: autumn Suspendid load Low Variable Bedload Low and fine Commonly high, coarse Water flow Meanly vertical Meanly horizontal Hydraulic vegetation effects maximums in summer and Commonly unimodal spring-summer of High Moderate-low Organic matter High Production Moderate-low CPOM (a) Low High FPOM (b) High Moderate-low DOM (c) High Low Biotic structure Continuum Communities or continuum (a) Coarse particulate organic matter (b) Fine particulate organic matter (c) Dissolved organic matter Table 3: Paraná river cross section. Species reachness in lotic and lentic environments near of the Confluence of the Paraná and Paraguay rivers. Channel (1) Island water bodies (2) (3) Ox-bow lake, fringe floodplain (4) CYANOPHYTA 19 13 12 CHLOROPHYTA 91 83 68 BACILLARIOPHYCEAE 57 48 43 CHRYSOPHYCEAE 8 7 4 XANTHOPHYCEAE 2 6 16 EUGLENOPHYTA 24 70 85 CRYPTOPHYCEAE 4 7 8 DINOPHYCEAE 2 4 6 Total de especies 207 238 242 (1) Zalocar de Domitrovic, Y. y E.R. Vallejos, 1982. Ecosur, 9(17): 1-28. (2) Zalocar de Domitrovic, Y., 1990. Ecosur, 16(27): 13-29. (3) Zalocar de Domitrovic, Y., 1992. Rev. Hydrobiol. Trop., 25(3): 177-188. (4) Zalocar de Domitrovic, Y., 1993. Ambiente Subtropical, 3: 39-67. Table 4: NET PRIMARY PRODUCTIVITY (ON DRY WEIGHT BASE) IN PARANA RIVER FLOODPLAIN, DOWNSTREAM OF PARANA-PARAGUAY CONFLUENCE. (After Neiff, 1990 b) Stress Period Long. (days) N.P.P. Stress Period (Tn.Ha-1) N.P.P. Annual Period (Tn.Ha-1) Production Rate (g.m-2.d-1) N.P.P. Effective Annual(Tn.Ha.-1.yr1) Production Rate (g.m-2.d-1) Polygonum ferrugineum 14 2.50 65.18 17.85 18.30 5.10 Ludwigia peploides 11 1.96 65.04 17.81 6.7 1.84 Victoria cruziana 10 0.29 10.58 2.90 0.89 0.24 Nymphoides indica 10 0.85 31.02 8.50 2.20 0.60 Echinochloa polystachya 12 1.72 52.31 14.33 14.10 3.86 Species FLOOD PERIOD Potamophase References: (*) P = Pond BW = Backswamp IS = Island LOW-WATER PERIOD Limnophase Site (*) Year El Gato (IS) 1987 La Guardia (Sta.Fe) (BW) 1971 Baupé (Chaco) (P) 1976 Don Felipe (Sta.Fe) (P) 1971 La Cacerola (Sta.Fe) (P) 1971 Fig. 2 (adapted from Zalocar, 1993): Comparison of phytoplankton diversity and similarity index before and after the flood in a pond of Paraná river floodplain. Fig. 5 (adapted from Marchese and Ezcurra de Drago, 1992): Alluvial valley of the Lower Paraná River: transverse zonation of the benthos in lotic environment. Fig. 6 (adapted from Poi de Neiff, 1989): Distribution of the relative abundance of invertebrate species associa- ted with aquatic vegetation. The mean monthly water level of Paraná River are given for Puerto Corrientes. No more Paraná water enters the floodplain below the level indicated by the horizontal dotted line.