2a, b and c scheme
advertisement

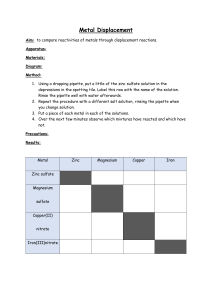
2a,b,c 1. Use periodic table Label groups and periods and metals and non-metals Practical (if you want but they will have done it in year 9) to look at electrical conductivity of metals and nonmetals. Properties of metals and non-metals Position of elements in the PT and their properties ( covered in 2b and 2c to a large extent) Group 0 and lack of reactivity 2. Reactions of group 1 with water – demo Na, Li and K. There is a film of Rb and Cs – I also do the word and symbol equations here and try to get them used to writing and balancing equations sooner rather than later! 3. Discuss and give notes on relative reactivity of group 1 elements and electron configuration. 4. For group 7 I usually start off by showing them the 3 elements - Cl2, Br2 and I2 and then reacting them with iron wool. Chlorine has to be made in situ. This leads onto the physical and chemical properties of the other halogens. 5. Hydrogen chloride and hydrochloric acid. This is quite difficult as we go over it again in bonding and acids and bases but needs to be covered. Practical HCl in water and methylbenzene. Does not work very well! 6. Reactivity and displacement Use chlorine water, bromine water and iodine water to displace halogens from salts – KI, KCl and KBr. You can also use cyclohexane to show the colour change better!