11.9A Polar Water - Texarkana Independent School District
advertisement

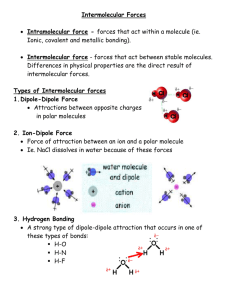
Focus Plan Texarkana Independent School District PLAN CODE: WRITER: IPC – 5th six weeks, Biology – 1st six weeks, Chemistry – 4th six weeks L. Petty COURSE/SUBJECT: 11th grade science GRADE(S): 11th TIME ALLOTTED FOR INSTRUCTION: 1 hour GRADING PERIOD: TITLE: Polar Water LESSON TOPIC: Solubility in polar and nonpolar solvents. TAKS OBJECTIVE: Objective 4 The student will demonstrate an understanding of the structures and properties of matter. 11.9 The student knows how solution chemistry is a part of everyday life. The student is expected to: (A) relate the structure of water to its function [as the universal solvent]. Objective 1: The student will demonstrate an understanding of the nature of science. 11.1 The student, for at least 40% of instructional time, conducts field and laboratory investigations using safe, environmentally appropriate, and ethical practices. The student is expected to: (A) demonstrate safe practices during field and laboratory investigations 11.2 The student uses scientific methods during field and laboratory investigations. The student is expected to: (A) plan and implement investigative procedures including asking questions, formulating testable hypotheses, and selecting equipment and technology (B) collect data and make measurements with precision (C) organize, analyze, evaluate, make inferences, and predict trends from data (D) communicate valid conclusions FOCUS TEKS AND STUDENT EXPECTATION: SUPPORTING TEKS AND STUDENT EXPECTATIONS: CONCEPTS Universal ENDURING UNDERSTANDINGS/GENERALIZATIONS/PRINCIPLES The student will understand that Solubility is a universal necessity. Life Living things cannot survive without certain compounds. Solubility These necessary compounds are soluble so they can be transported through the body. Some compounds are soluble in water and some are only soluble in other types of solvents. The polarity of water allows more types of substances to dissolve in water than in any other solvent. Solvents Polarity I. SEQUENCE OF ACTIVITIES (INSTRUCTIONAL STRATEGIES) A. Focus/connections/anticipatory set When students are seated, show a short clip of blood rushing through the veins or arteries. This can be found on any video dealing with the circulatory system. When the video has played for several minutes, turn it off and explain to students that the blood is necessary to transport materials throughout the body. If this transportation didn’t work, they would not live. In order to get through the body, however, these materials must first dissolve in the blood; they must be soluble. Explain that this lab deals with the solubility of substances. B. Instructional activities (demonstrations, lectures, examples, hands-on experiences, role play, active learning experience, art, music, modeling, discussion, reading, listening, viewing, etc.) 1. Lecture Go over Transparency master – Vocabulary Go over Transparency master – Explaining Polarity C. Guided activity or strategy Have a water molecule and a salt molecule (either with paper models or wood models) to demonstrate how a polar solvent helps pull a polar molecule apart. Hold the oxygen side of the water (negative) next to the sodium (positive side of the salt) and explain that the negative and positive charges form a slight attraction. This attraction can interfere with the attraction between the original molecules and cause them to dissolve. D. Accommodations/modifications Students requiring accommodations may be given copies of both transparency masters and may be assigned a peer tutor. E. Enrichment Students requiring enrichment may define the vocabulary prior to class for a grade and may be assigned as a peer tutor. II. STUDENT PERFORMANCE A. Description Students should complete Lab – Polar Water. B. Accommodations/modifications Students requiring accommodations may be assigned a peer tutor. C. Enrichment Students requiring enrichment should be assigned as a peer tutor. III. ASSESSMENT OF ACTIVITIES A. Description Complete Lab Worksheet – Polar Water B. Rubrics/grading criteria Deduct 3 points for each data table box answered incorrectly or left blank. Each part of each question is worth 5 points (25 total). C. Accommodations/modifications None needed. D. Enrichment Students requiring enrichment should be asked to differentiate between miscible and soluble. They may also be given more substances to investigate or research to determine their solubility in water. If more lab time is desired, they may be given the assignment to test the solubilities in another substance like alcohol. E. Sample discussion questions 1. 2. 3. IV. Which substance was the most soluble in water? This will depend on your lab, but will often be sucrose or ethanol. Which substance was the least soluble in water? Again, this will depend on the lab, but will often be glycerol or kerosene. Alcohol is a nonpolar molecule, which types of substances should dissolve in alcohol? The rule for dissolving is generally “like dissolves like”, so alcohol should dissolve nonpolar substances. TAKS PREPARATION A. Transition to TAKS context 1. The polar structure of water molecules enables it to act as an effective solvent for ionic compounds. Why does this occur? (a) The polar structure of water molecules reduces the electrostatic attraction between positively charged ions and negatively charged ions in the solute. (b) The polar structure of water molecules increases the electrostatic attraction between positively charged ions and negatively charged ions in the solute. (c) The polar structure of water molecules is weakened by positively charged ions and negatively charged ions in the solute. (d) The polar structure of water molecules is strengthened by positively charged ions and negatively charged ions in the solute. 2. Water is an effective solvent for ionic compounds because the polar structure of its molecules reduces the electrostatic attraction between ions having positive charges and negative charges. The ions become hydrated, or surrounded by water molecules. Why do the ions remain separate rather than recombining to come out of solution? (a) The ionic charges are distributed within a smaller structure. (b) The ionic charges are concentrated within a smaller structure. (c) The ionic charges are distributed within a larger structure. (d) The ionic charges are concentrated within a larger structure. B. Sample TAKS questions Spring 2003 1. If the properties of water were to change so that the solid form was denser than the liquid form, organisms living in a cold pond environment would be less likely to survive because water would no longer ____. (a) dissolve enough oxygen from the air (b) produce solutions containing vital nutrients (c) remain neutral, instead becoming highly acidic (d) produce a floating insulating layer of ice Spring 2004 2. Fish survive through severe winters because of the property of water that allows water to ____. (a) form chemical bonds as it freezes, raising the water temperature below the ice (b) increase in density while it freezes, dissolving more oxygen from the air (c) expand when it freezes, creating a floating and insulating layer of ice (d) precipitate vital nutrients when it freezes, increasing the food supply V. KEY VOCABULARY insoluble miscible nonpolar VI. polar solubility soluble RESOURCES A. Textbook – none needed. B. Supplementary materials/equipment Transparency master – Explaining Polarity Transparency master – Vocabulary Instructor’s Copy - Vocabulary Lab Instructions – Polar Water Lab Worksheet – Polar Water Instructor’s Copy – Polar Water C. VII. Technology FOLLOW UP ACTIVITIES (reteaching, cross-curricular support, technology activities, next lesson in sequence, etc.) A. Reteaching Go over graded labs with students. B. Next lesson in sequence All subjects – solubility. VIII. TEACHER NOTES Before lab: 1. Make sure all containers are properly labeled. 2. Make sure liquid chemicals are in dropper bottles. 3. Have a disposal container for the hexane and kerosene. 4. Caution students that the sample sizes used for solid chemicals must be the same or they will invalidate their lab results. During lab: 5. Make sure students are using small, even amounts of the solid chemicals. 6. Check their technique for “flicking” 7. Make sure students do not dump hexane and kerosene down the drain.