4.1.2 Aldehydes and Ketones Booklet
advertisement

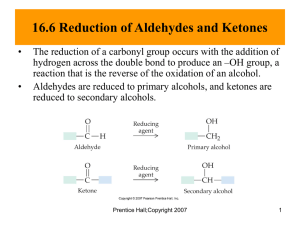
Aldehydes and ketones contain the carbonyl functional group. The functional group is the part of a molecule which takes part in chemical reactions. Lots of structures contain the carbonyl group, so what distinguishes aldehydes and ketones? Aldehydes have the carbonyl group on a carbon with at least one hydrogen atom attached to it and ketones have the carbonyl group attached to two alkyl groups. Student Activity Name each of the following functional groups. 1 Naming Aldehydes In aldehydes, the carbon atom of the group is joined to at least one hydrogen atom. They are named using the suffix –al. The prefix is given by the number of carbons. O H3C As the aldehyde group is on the end of the chain it is not actually stated in the name. C The compound is called ethanal. H Naming Ketones In ketones, the carbon atom of the group is joined to two other carbon atoms. The parent chain is the longest chain containing the C=O. They are named using the suffix –one. O H3C C H C H CH3 As the carbonyl group is the second carbon in a chain containing four carbons, it is numbered 2. The compound is called butanone. (The number position of the C=O group in ketones needs to be specified for carbon chains of over 4, or less, if substituents are present e.g. 3methylbutan-2-one). Student Activity 1.Name each of the following carbonyl compounds. a) _______________________ CH3CH2CH2CH2CH2CH2CH2COCH2CH3 O b) C3H7 _________________________ C H O c) C2H5 _________________________ C C2H5 d) _______________________ e) __________________________ 2 2. Draw displayed and skeletal formula for each of the following a) propanal b) 1-chlorobutan-2-one c) 3-bromo-2-methylpentanal Preparation of Aldehydes and Ketones You should recall that these are made by oxidation reactions of alcohols. Student Activity (questions below) 1. Label the molecules above as primary (1o), secondary (2o) or tertiary (3o) alcohols. 2. Which of the following can be oxidised by acidified dichromate (tick the ones which react)? i. propan-1-ol ii. 2-methylpropan-2-ol 3 iii. butan-2-ol iv. 2-methylpropan-1-ol v. cyclohexanol 3. State the colour change for the reactions which occur above. ____________________________________________________ 4. Why can’t tertiary alcohols be oxidised easily? ____________________________________________________ Oxidation Alcohols can be oxidised to aldehydes and ketones. Writing equations for oxidations We write oxidation equations using [O] to represent the oxidising agent. Water is produced on the right hand side of the equation. Student Activity Write equations for the oxidation of the following alcohols (using structural formulae). 1. butan-2-ol ___________________________________________________ 2. propan-1-ol ___________________________________________________ The oxidation can go a stage further with primary alcohols because aldehydes are also oxidisable. We have to control the conditions carefully to get the product we want: Reflux – gives the carboxylic acid Distillation – gives the aldehyde 4 Reduction of Aldehydes and Ketones Carbonyl compounds are reduced to alcohols using a suitable reducing agent such as NaBH4 (sodium tetrahydridoborate) in an ethanol solvent Aldehydes Ketones primary alcohols secondary alcohols [H] is used for a reducing agent rather like [O] for oxidation above. Student Activity Write equations for the reduction of the following carbonyls. a) propanone ________________________________________________________ b) propanal ________________________________________________________ c) 2-methylhexan-3-one ________________________________________________________ Note: describe the pi bond in a carbonyl compound and why it doesn’t react with electrophiles (HW4 Q1) Nucleophilic Addition Reactions The carbonyl group in the aldehyde and ketone is polar. This makes the carbon atom susceptible to attack by nucleophiles. The carbonyl group is unsaturated as it contains a carbon to oxygen double bond and it undergoes addition reactions. The reaction is called nucleophilic addition. There is only one product. Nucleophile? What’s one of them? Mechanism for the reaction between butanone and the hydride ion This reduction (adding of hydrogen, removal of oxygen) takes place by the addition of the hydride ion across the carbonyl group followed by the protonation, by water, of the intermediate organic species produced. 5 Reaction with 2,4-dinitrophenylhydrazine (2,4-dnph) (Brady’s reagent) When a solution of 2,4-dinitrophenylhydrazine is added to an aldehyde or a ketone, a deep yellow or orange solid is made. This precipitate is called a 2,4-dinitrophenylhydrazone (2,4–dnp derivative). The test is specific for an aldehyde and a ketone. There is no precipitation with a carboxylic acid or with an ester – although both of these classes of compounds contain the carbonyl. The test can be used to identify precisely the name of the compound as explained below. Below is an equation for the reaction of butanal. You don’t need to learn the structure of 2,4 dnph. H NH2 O C3H7 C + O2 N NH N O2N NH C + H2O C3H7 H NO2 NO2 2,4-dinitrophenylhydrazine 2,4-dinitrophenylhydrazone The product of this reaction is a solid and is usually slightly impure at this stage. Recrystalisation is carried out to produce fine yellow or orange crystals. The melting point of this dry crystalline solid is determined and recorded. The value is then compared to a data table and it is possible to identify the original aldehyde or ketone. Test to distinguish between an aldehyde and a ketone Aldehydes are easily oxidised to carboxylic acids by mild oxidising agents, whereas ketones are not. A weak oxidising agent, which can be used to distinguish between the aldehyde and the ketone, is Tollens’ reagent. Tollens’ reagent can be made easily in the laboratory by adding sodium hydroxide to silver nitrate until a brown precipitate is formed and then adding dilute ammonia until the precipitate just dissolves. This colourless solution is Tollens’ reagent; it contains a silver complex ion which can be reduced by the aldehyde to silver metal. Tollens’ reagent is sometimes called ammoniacal silver nitrate. Carbonyl Compound Aldehyde Ketone Observation Silver Mirror is formed No Reaction The oxidising species in Tollen’s reagent are the aqueous silver(I) ions, Ag+(aq). When Tollen’s reacts with the aldehyde; 6 The aldehyde is oxidised to a carboxylic acid; O C4H9 O + [O] C C4H9 C H OH The silver ions are reduced to silver metal. Ag+(aq) + e- Ag(s) Student activity Teacher note: draw the structures. Complete the following table, with a tick if it reacts and a cross if it doesn’t. Compound Reaction with Br2 at room temperature Reaction with hot Na2Cr2O7/H+ pentan-3-one butan-2-ol pentanal ethene 2-methyl propan2-ol 7 Reaction with Tollens’ reagent 8 Experiment 1: Ketones Reactions of Aldehydes and Risk: Hazard category B: Great care needed. Read all hazard warnings carefully. Toxic, poisonous, corrosive and explosive substances are all involved. Wear goggles and lab coat. Mop up spills using water. Wash hands thoroughly afterwards. Watch out for toxic and explosive substances and follow all instructions to the letter. You are provided with separate samples of A and B, one of which is an aldehyde and the other a keone. In pairs, carry out the following tests to identify them – divide up the work between you! Prepare a results table. 1. To confirm aldehydes or ketones, add 2 cm3 of 2,4 dnph solution (Brady’s reagent – highly corrosive and toxic) to each of three test tubes and then add 5 drops of unknown liquid. A positive result is orange/red crystals. 2. Silver mirror test To approximately 1 cm3 of a solution of silver nitrate add 1 drop of aqueous sodium hydroxide to make a precipitate. Add aqueous ammonia dropwise until the precipitate just re-dissolves – this is called TOLLENS’ reagent and must be disposed of immediately after use as it explodes on standing! Make up two tubes of this reagent. Add 5 drops (separately) of the unknown liquids to your tubes and warm it in a water bath of boiling water. A positive result is a silver mirror and this means you have an aldehyde present. 3. Fehling’s test Add Fehling’s B to 1 cm3 of Fehling’s A until the blue precipitate just redissolves to give a deep blue solution. Prepare four tubes of this mixture. Then add 5 drops of unknown liquid to the tube and place in the boiling water bath. A positive result is a brick red precipitate and this means you have an aldehyde present – but it does not work for benzaldehyde. 9 Experiment 3: Purification and Identification of an Organic Compound Risk Class C: Some risk involved where great care must be taken because of the nature of the chemicals or equipment involved. Students need to be warned of the dangers and trained to avoid risks. Wear goggles and lab coat. Mop up spills using water. Wash hands thoroughly afterwards. The organic substance is an irritant! You are provided with an impure organic compound – it is contaminated with an insoluble black solid and a soluble dye. You will purify it and show its purity by measuring the melting point – a pure substance melts at a sharp, fixed temperature. 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Weigh accurately about 3 g of solid. Dissolve it in the MINIMUM quantity of hot distilled water – have both the solution and solvent on the same Bunsen. Filter the hot, saturated solution through a pre-heated funnel and flask using fluted filter paper, discard the residue. Allow the filtrate to cool slowly and form crystals. Filter the crystals using a Buchner flask and funnel under reduced pressure. Wash out the original conical flask and rinse the crystals with cold distilled water. Dry the crystals between filter papers. Weigh a weighing bottle then transfer the crystals to the bottle and re-weigh. Work out your % yield. Label the bottle with your name, mass of sample and yield. Mass of bottle = Mass of bottle + crystals = Mass of crystals = % yield (show working) = NEXT LESSON Take a rough ( ) and an accurate ( a small sample of your crystals. 10 )melting point of Experiment 4: Determination of Melting Point The purity of a substance prepared for example by recrystallisation can be checked by measuring its melting point and comparing this with the book value. Remember A pure substance melts at a fixed and sharp temperature Impurities lower and broaden the melting point Method 1. Thoroughly dry a small sample of your substance by pressing between filter papers 2. Force some crystals into the open end of the melting point tube, invert, gently tap the bottom so the solid falls to the bottom – you do not need very much! 3. Place the tube into one of the three holes in the machine 4. 5. 6. 7. Turn the machine on, turn the boost on and turn the dial until the sample starts to melt. Switch off the boost and record the exact temperature at which liquid appears. This is a rough measurement. Repeat but more slowly near the melting point. You will probably find the melting point is lower than your rough one. It is possible to do more than one measurement simultaneously – use all three holes if necessary! Make sure you use the same machine each time and that you stick with either the digital read out or the thermometer reading. 11 Experiment 5: Reducing a ketone using NaBH4 Risk: Class B This experiment needs close supervision by the teacher because there are significant risks due to the nature of the chemicals or equipment used. Wear goggles and lab coat. Mop up spills using water. Wash hands thoroughly afterwards. Read the hazard warning labels carefully and treat accordingly. Do not carry out the experiment until you have the permission and attention of your teacher. NaBH4 is properly known as sodium tetrahydroborate(III); it is commonly called sodium borohydride. The method involves dissolving the carbonyl compound in warm ethanol (contains about 10 % water). The NaBH4 is added, the mixture stirred and left for 10 to 15 minutes – heat is usually evolved. Water is added and the mixture boiled - this destroys the excess NaBH4. These reactions happen in 2 steps: a) Step 1: initiated by the nucleophilic attack of an H- to the C of the carbonyl group, b) Step 2: followed by the attack of a H+ ion from water to the anion. Overall: R1COR2 + 2 H ( H- + H+ ) NaBH4 R1CH(OH)R2 H2O / heat The mechanism is: 1) Add 0.7g of 1,2-diphenylethanedione (Benzil) to 7cm3 of ethanol in a small conical flask 2) Warm the mixture in a hot water bath at about 50oC until the solid dissolves. 3) Cool. When the solid reappears as fine suspension add 0.15g sodium tetahydroborate (III). Allow to stand for 10 minutes. 4) Add 15cm3 of distilled water and heat until the water boils. Allow the mixture to cool. 5) Collect the crystals by suction (Buchner) filtration. Suck dry and wash with 100cm3 of distilled water. Transfer the solid to a clean filter paper and press dry. 6) Design some chemical experiments to show that the starting material and the product have different functional groups. 7) Write down a balanced equation using displayed formulae and 2H as the reductant. 12 13