Isotopes – Atoms of the same element with different masses The
advertisement

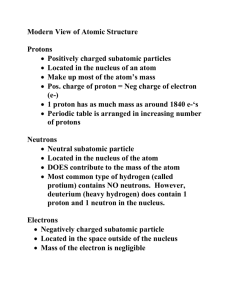
Isotopes – Atoms of the same element with different masses The difference in mass is due to the atoms having different numbers of neutrons Example: Hydrogen (Atomic Number = 1) has 3 isotopes, a specific isotope is called a nuclide. Protium 1 p+, 0 no, 1 e- mass = 1 (Hydrogen-1) 1 1𝐻 Deuterium 1 p+, 1 no, 1 e- mass = 2 (Hydrogen-2) 2 1𝐻 Tritium 1 p+, 2 no, 1 e- mass = 3 (Hydrogen-3) 3 1𝐻 Nuclear or Isotopic Symbol (Same number of Protons therefore same element) The isotopes of an element have similar chemical and physical properties, but different nuclear properties. A Nuclear or Isotopic Symbol is used to indicate a specific nuclide and has the format: 𝑀𝑎𝑠𝑠 𝑁𝑢𝑚𝑏𝑒𝑟 𝐴𝑡𝑜𝑚𝑖𝑐 𝑁𝑢𝑚𝑏𝑒𝑟 𝐶ℎ𝑒𝑚𝑖𝑐𝑎𝑙 𝑆𝑦𝑚𝑏𝑜𝑙 Knowing the nuclide allows us to determine the number of electrons, protons and neutrons in an atom. Atomic Number = the number of protons In a neutral atom the number of protons = the number of electrons Mass Number = number of protons + number of neutrons (The unit for Mass Number is understood to be amu) The number of neutrons = Mass Number – Atomic Number Example 1: 41 20𝐶𝑎 Mass Number = 41 Atomic Number = 20 20 p+, 20 e-, 21 no Example 2: An atom with 34 p+ and 45 no would be: Selenium Atomic Number = 45 Mass Number = 34 + 45 = 79 Electrons = 34 Nuclear Symbol is 79 34𝑆𝑒 Example 3: Uranium – 235 Atomic Number = 92 Mass Number = 235 Protons = 92 Electrons = 92 Neutrons = 235 – 92 = 143 Nuclear Symbol is 235 92𝑈 Relative Mass The masses given on the periodic table are the weighted average masses of the naturally occurring isotopes of each element compared to the mass of the Carbon – 12 atom. Because of the mass defect (mass converted to energy in the formation of the atom) and the slight difference in the mass of a proton and neutron, and the mass of the electrons, the actual mass of an atom is not exactly equal to the sum of the masses of the particles in the atom. The mass we use is a Relative Mass. A Relative Mass is a mass compared to some standard. The standard used for atomic mass is the Carbon-12 nuclide. The mass of Carbon – 12 is defined as being exactly 12 amu. 1 Atomic Mass Unit is defined as being 1/12 the mass of the Carbon-12 atom which is approximately equal to 1.66 x 10-24 g. A naturally occurring sample of an element will contain a mixture of isotopes of that element. The proportions of these isotopes are fairly consistent, regardless of the source of the sample. These proportions are used to calculate a weighted average for the mass of the element, called the atomic mass or atomic weight. This is the mass given on the Periodic Table. When we are working with a sample the atomic mass is measured in g/mol, when working with a specific atom the atomic mass is measured in amu/atom. Example: there are 3 naturally occurring isotopes of Silicon. The proportions (Relative Abundance) for each are given here along with their masses Isotope Relative Abundance Mass Silicon – 28 92.23% 27.97693 amu Silicon-29 4.67% 28.97649 amu Silicon-30 3.10% 29.97376 amu The Periodic Table gives the Atomic Mass of Silicon as 28.0855, which is fairly close to the mass of the most commonly occurring isotope. There are 2 ways to find the Average Atomic Mass 1) Multiply the mass of each isotope by its relative abundance, add these products and then divide by 100 2) Multiply each isotope’s mass by the decimal form of the percentage and the values of these products are added together. The decimal form of the percentage is called the Fractional Abundance. The calculations for Silicon would be: 1) Isotope Relative Abundance Mass Calculation Silicon – 28 92.23% 27.97693 amu 2580.312254 Silicon-29 4.67% 28.97649 amu 135.3202083 Silicon-30 3.10% 29.97376 amu 92.918656 2808.551118/100 = 28.0855 2) (27.97693 amu x 0.9223)+ (28.97649 amu x 0.0467) + (29.97376 amu x 0.0310) = 28.0855amu/atom or g/mol Example 2: Uranium has 3 isotopes with the following percentages and masses U-234 234.040947 amu at 0.005% U-235 235.043924 amu at 0.720 % U-238 238.050784 amu at 99.275% Atomic Mass = (234.040947 amu x 0.00005) + (235.043924 x 0.00720) + (238.050784 x 0.99275) = 238.0289341 amu/atom or g/mol Example 3: Element X has 3 isotopes. X – 24 has a relative abundance of 78.99% and a mass of 23.98504 amu, X – 25 has a relative abundance of 10.00 % and a mass of 24.98584 amu and X – 26 has a mass of 25.98259 with an unknown relative abundance. A) Find the relative abundance of the X – 26 isotope. 100 – 78.99 – 10.00 = 11.01% B) Find the average atomic mass of this element. ((78.99 X 23.98504) + (10.00 x 24.98584) + (11.01 x 25.98259))/100 = 24.30505 amu/atom C) Identify the element Magnesium