Enantioselective hydrolysis of racemic styrene oxide and its
advertisement

Supplementary Information for Biotechnology Letters [BILE-D-13-00914R1]
Enantioselective hydrolysis of racemic styrene oxide and its
substituted derivatives using newly isolated Sphingopyxis sp.
BSNA05 exhibiting a novel epoxide hydrolase activity
Jung-Hee Woo1,*, and Eun Yeol Lee2,*
1
Gyeongbuk Institute for Marine Bio-Industry (GIMB), Uljin 767-813, Gyeongbuk, Republic
of Korea
2
Department of Chemical Engineering, Kyung Hee University,
Gyeonggi-do 446-701, Republic of Korea
*To whom correspondence should be addressed
Tel: 82-54-780-3454, Fax: 82-54-780-3469, E-mail: jhwoo@gimb.or.kr
Tel: 82-31-201-3839, Fax: 82-31-204-8114, E-mail: eunylee@khu.ac.kr
1
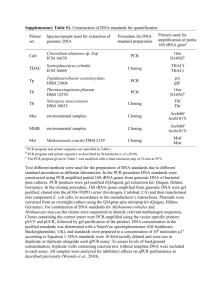
Supplementary Table 1. Parameters of enantioselective hydrolysis of racemic styrene oxide
(SO) by the strain 8-NA-5.
Yield
Epoxide
Time (h)
ee (%)
ca
Eb
4 mM SOe
13
86.46
0.8039
3.9
21.8
S
4 mM SOf
7
99.99
0.7606
12.2
20.6
S
(%)c
Abs. conf.d
a. The extent of conversion (c) [c ={1−(ERs+ESs/ERso+ESso)}], where the initial concentrations
of (R)- and (S)-epoxides are denoted as ERso and ESso, respectively, and the concentrations
of the remaining (R)- and (S)-epoxides are denoted as ERs and ESs, respectively.
b. The enantiomeric ratio (E-value) was derived from the extent of conversion (c) and the
enantiomeric excess of the remaining substrate enantiomers (ees) [E= In{(1−c)
(1−ees)}/In{(1−c) (1+ees)}]
c. Yield of the remaining epoxide
d. Absolute configuration of the remaining epoxide
e. Using whole cells (dry weight, 5 mg)
f. Using whole cells (dry weight, 10 mg)
2
Supplementary Table 2. Enantioselective EHase activity of Sphingopyxis sp. BSNA05
toward styrene oxide and its derivatives.
Hydrolysis rate
(x 10-2) mg/hour
Strains
Sphingopyxis
sp.
BSNA05
(8-NA-5)
SO
2CSO
3CSO
4CSO
(R)
(S)
(R)
(S)
(R)
(S)
(R)
(S)
1.48
0.50
1.76
0.51
0.87
0.08
0.97
0.34
3
Isolation of microbial consortium and single colonies from PAH–based enrichment
culture
A 0.5 g mud sample from the oil-spilled foreshore was suspended in 5 mL MSM
with PAHs (naphthalene, phenanthrene, pyrene and benzopyrene) at 25 oC for 50 days. Then,
1 mL of the enriched culture was re-suspended in fresh MSM with PAHs for two weeks. A
microbial consortium was obtained from the enriched culture, and then many single colonies
possessing PAH-degrading activity were isolated from the consortium. Microbial strains
possessing EHase activity were identified from the isolated colonies based on GC analysis for
kinetic resolution of racemic styrene oxide using whole cells.
Marine broth medium (Difco) and mineral salt medium (MSM) (4 g NaNO3 l-1; 1.5 g
KH2PO4 l-1; 0.005 g FeCl36H2O l-1; 0.2 g MgSO4 l-1; 0.01 g CaCl2H2O l-1; 0.5 g Na2HPO4 l-1;
pH 7.2) were used in this study. Microorganisms were enriched using polycyclic aromatic
hydrocarbon (PAH) and isolated from the samples of the oil-spilled foreshore of South Korea
(Yeosu, Byeonsan, Gunsan and Taean).
Phylogenetic analysis of microbial strains using 16S rRNA sequencing
Genomic DNA of the isolated strain was isolated using a Wizard Genomic
DNA Purification Kit (Promega, Madison, WI, USA). The 16S rRNA was amplified using
16S rRNA primers, 27F (5'-AGA GTT TGA TCM TGG CTC AG-3'; Escherichia coli
nucleotide 8~27) and 1518R (5'-AAG GAG GTG ATC CAN CCR CA-3'; E. coli nucleotide
1541~1522), from genomic DNA. PCR of 16S rRNA was conducted using PCR buffer
containing 50 mM Tris-HCl (pH 9.0), 0.1 % TritonX-100, 1.5 mM MgCl2, 0.2 mM dNTPs,
4
0.2 μM primers, 2.5 U Taq DNA polymerase (Promega), and 2.5 - 250 ng template in 50 μL.
DNA amplification was conducted with a PE 2400 thermal cycler (PE Applied Biosystem,
Foster City, California, USA). PCR conditions were as follows: initial denaturation at 94 oC
for 5 min and 35 cycles of denaturation (94 oC, 1 min), annealing (60 oC, 1 min), extension
(72 oC, 2 min), and then 7 min incubation at 72
o
C. PCR products were separated using a
DNA Purification System (Wizard PCR Preps DNA Purification System, Promega), and then
were ligated into pGEM-T Easy Vector (Promega). The recombinant plasmids were
transformed into E. coli JM109 host. The 16S rDNA sequence was determined using an
automatic sequencer (ABI Prism 377 DNA Sequencer, Perkin Elmer, Waltham, MA, USA).
Bioinformatic analysis of 16S rDNA was conducted using SIMILARITY-RANK of the
Ribosomal Database Project (RDP), Basic Local Alignment Search Tool (BLAST) of the
National Center Biotechnology Information (NCBI) and Phylogenetic Interference Package
(PHYLIP, version 3.57c). Sequence similarity was analyzed using the DNADIST program,
and a phylogenetic tree was constructed using the FITCH program.
5
Supplementary Fig. 1. Time-course of chiral GC analysis during enantioselective hydrolysis
of 4 mM racemic styrene oxide by Sphingopyxis sp. BSNA05.
6
Supplementary Fig. 2. 16S rRNA sequence-based phylogenetic tree of the strain 8-NA-5
possessing epoxide hydrolase activity. The numbers within brackets indicate accession
numbers from the GenBank data library.
7