Supplementary Information (doc 38K)
advertisement

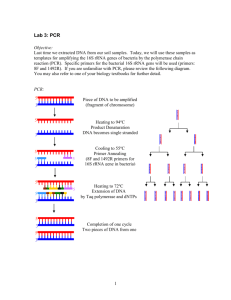
Supplementary Information: DNA/RNA extraction - DNA and RNA were extracted from 3 replicates of each sample using half of each minicore (0.5 cm2; top 2 mm approx 0.25 g dry weight) by beadbeating, using a Mikro-dismembrator-U (B. Braun, Biotech International) at 2000 rpm. for 30 s, in 2 ml microcentrifuge tubes containing 1 g of 0.1 mm Zirconia/Silica beads (Biospec products Inc.), 0.5 ml of 1 M sodium phosphate buffer (pH 8.0), and 0.5 ml of phenol-chloroform-isoamyl alcohol (25:24:1). After centrifugation (16,100 g for 1 min at 4°C), 450 ml of the upper aqueous layer was removed and added to tubes containing 0.03 g polyvinylpolypyrrolidone (Sigma), and incubated at 4oC for 1 h to remove inhibitory humic acids. After centrifugation the supernatant was transferred to tubes containing 1 volume of chloroform-isoamyl alcohol (24:1) and mixed by inversion. After further centrifugation (16,100 g for 1 min at 4°C), the upper aqueous layer was transferred to a clean tube and nucleic acids were precipitated on ice for 30 min by adding 0.1 volume of 3 M sodium acetate (pH 5.2) and 2.5 volumes of absolute ethanol. Nucleic acids were pelleted by centrifugation (16,100 g for 25 min at 4°C), washed with 70% ethanol and air-dried before resuspending in 200 l of DEPC-treated water. DNA was removed from 50 l of the total nucleic acid extraction by DNase digestion, using Turbo DNA-free™ protocols (Ambion) and a control PCR was performed to confirm that DNA was completely removed. Reverse transcription polymerase chain reaction (RT-PCR) - Reverse transcription was performed using SuperScript™ reverse transcriptase III (Invitrogen) using protocols as recommended by the manufacturer with 0.1 mM reverse primer (described below). 2 l of reverse transcription products were subsequently used in PCR reactions to amplify the variable V3 region of the 16S rRNA gene (Escherichia coli positions 341–534) using primers to conserved regions (forward primer with GC clamp 5′ - CGC CCG CCG CGC GCG GCG GGC GGG GCG GGG GCA CGG GGG GCC TAC GGG AGG CAG CAG - 3′; reverse primer 5′ - ATT ACC GCG GCT GCT GG - 3′) (Muyzer et al, 1993). Separate reactions were also performed with Archaea-specific 16S rRNA primers ARC344F (forward primer with GC clamp 5′ - CGC CCG CCG CGC CCC GCG CCC GTC CCG CCG CCC CCG CCC GAC GGG GYG CAG CAG GCG CGA - 3′) (Stahl and Amann, 1991) and ARC915R (5′ GTG CTC CCC CGC CAA TTC CT - 3′) (Raskin et al, 1994). 50 l reactions contained 0.4 mM of forward and reverse primer, 0.1 mM dNTPs, 2.5 U of Taq polymerase and 5 ml of the reaction buffer supplied with the enzyme (Qiagen®), and 2 ml of template cDNA. Amplification was performed in a Gene Amp® PCR system 9700 Thermocycler (Applied Biosystems) as follows: 95°C for 30 s, 30 cycles of 94°C for 30 s, 60°C for 30 s and 72°C for 30 s, then 72°C for 10 min. Amplification of the target cDNA was confirmed by agarose gel electrophoresis (2% w/v in 1 TAE buffer at 100 V) and ethidium bromide staining. Denaturing gradient gel electrophoresis - Denaturing gradient gel electrophoresis analysis of bacterial and archaeal 16S rRNA RT-PCR products was performed using the Bio-Rad D Code system, on gels consisting of 8% (w/v) polyacrylamide (acrylamide: bisacrylamide, 37:1) and a denaturing gradient from 40% to 60% [100% denaturant is 7 M urea and 40% v/v formamide], in 1 TAE (40 mM Tris-acetate, 1 mM di-sodium-EDTA, pH 8.0), at 60V and 60°C for 17 h. After electrophoresis, gels were stained with silver nitrate. DGGE gels were analysed for relative band intensity using Phoretix 1D Version 5.00. Triplicate DGGE profiles per sample were imaged, all of which were highly similar. These triplicates were then pooled (5l RT-PCR product from each replicate) and the combined 15l used to produce the composite DGGE gel presented in Fig.4. Q-PCR – Bacterial and Archaeal 16S rRNA gene abundance was quantified by separate Q-PCR reactions on an ABI 7000 Sequence Detection System (Applied Biosystems), using the primer pairs (but without the GC clamps) detailed in the DGGE section. DNA isolated from the sediment minicores was amplified in triplicate together with no-template controls. Reaction mixtures using SYBR® Green PCR Master Mix (Applied Biosystems), cycling conditions, standard curve construction (R2=0.99, E=95%), and quantification were performed as described previously (McKew et al, 2007). 16S rRNA PCR amplicon libraries - Bacterial community composition and diversity were assessed from 16S rRNA gene libraries constructed from the DNA extracts of selected samples (Wet controls from days 1 and 28, desiccated samples from day 14 and 23, and the reflooded sediment samples from day 28) using Roche 454 pyrosequencing technology at the NERC Molecular Genetics Facility at the University of Liverpool. Amplicons for the pyrosequencing libraries were produced by PCR amplification of the V3 region of the 16S rRNA gene using the same target sequence and PCR cycling conditions as detailed in the RT-PCR section. Samples were multiplexed on a 1/8 section of the pyrosequencing plate, so fusion primers also contained a unique 10 base barcode to distinguish each sample (Parameswaran et al, 2007). Sequences analysed were a minimum length of 150 base pairs (mean length 183 base pairs) and were classified using the Greengenes database (DeSantis et al, 2006b) and NAST multiple sequence aligner (DeSantis et al, 2006a). Phylogenies were constructed with the PHYLIP software package (Felsenstein, 2005), using the neighbour-joining algorithm, and analysed with UNIFRAC (Lozupone et al, 2006). Shannon diversity scores (H′) were calculated using the RDP Pyrosequencing Pipeline’s alignment, complete linkage clustering and Shannon and Chao1 index tools. Supplementary references: DeSantis TZ, Hugenholtz P, Keller K, Brodie EL, Larsen N, Piceno YM et al. (2006a). NAST: a multiple sequence alignment server for comparative analysis of 16S rRNA genes. Nucleic Acids Res 34:W394-W399. DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K et al. (2006b). Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72:5069-5072. Felsenstein J. (2005). PHYLIP (Phylogeny Inference Package) version 3.6. Distributed by the author. Department of Genome Sciences, University of Washington, Seattle. Lozupone C, Hamady M, Knight R. (2006). UniFrac - An online tool for comparing microbial community diversity in a phylogenetic context. BMC Bioinformatics 7:371. McKew BA, Coulon F, Yakimov MM, Denaro R, Genovese M, Smith CJ et al. (2007). Efficacy of intervention strategies for bioremediation of crude oil in marine systems and effects on indigenous hydrocarbonoclastic bacteria. Environ Microbiol 9:1562-1571. Muyzer G, Dewaal EC, Uitterlinden AG. (1993). Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695-700. Parameswaran P, Jalili R, Tao L, Shokralla S, Gharizadeh B, Ronaghi M, Fire AZ (2007). A pyrosequencing-tailored nucleotide barcode design unveils opportunities for large-scale sample multiplexing. Nucleic Acids Res. 35:e130. Raskin L, Stromley JM, Rittmann BE, Stahl DA. (1994). Group-specific 16S rRNA hybridization probes to describe natural communities of methanogens. Appl Environ Microbiol 60:1232-1240. Stahl DA, Amann R. (1991). Development and application of nucleic acid probes. In E. Stackebrandt and M. Goodfellow [eds.], Nucleic acid techniques in bacterial systematics. John Wiley and Sons: New York. pp. 205-248.