Table 1 Biocontrol activity of Pseudomonas corrugata strain CCR04
advertisement

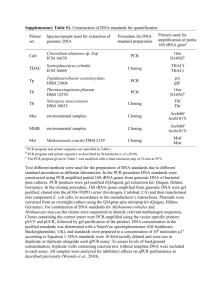
Supplementary Materials and methods Fatty acid methyl ester profiling Total cellular fatty acid-methyl ester (FAME) analysis of strains CCR04 and CCR80 was performed by gas chromatography (Hewlett-Packard 5898A, GC system, Avondale, PA, USA) with the Microbial Identification System (MIDI) (Newark, DE, USA) according to the manufacturer’s instructions. Morphological, biochemical and physiological identification The shape, cell size and flagella of strains CCR04 and CCR80 were examined using transmission electron microscopy (TEM) (LEO 912AB, LEO Electron Microscopy, Cambridge, England) as described by Kim et al. (2012). The phenotypic characteristics of strains CCR04 and CCR80 were compared with seven reference species of Pseudomonas, which were obtained from the Korean Agricultural Culture Collection (KACC) of the National Institute of Agricultural Biotechnology in the Rural Development Administration (Suwon, Korea). Gram staining; hydrolysis of gelatin, starch and Tween 80; production of H2S, catalase and acid from carbohydrate; growth on trypticase soy agar and nutrient agar supplemented with 1, 3 and 5 % NaCl; fluorescence production; glucose utilization were tested using the methods of Gerhardt (1994). Degradation of urea was determined using the procedures of Bowman et al. (1996). 16S rRNA gene sequence analysis Total genomic DNA of strains CCR04 and CCR80 was isolated using a QIAGEN Genomic-tip kit (Qiagen GmbhH, Hilden, Germany) according to the manufacturer’s instructions. The 1403-bp and 1400-bp DNA fragments encompassing the 16S rRNA genes of strains CCR04 and CCR80, respectively, were amplified by polymerase chain reaction (PCR) using universal primers: fD1 (5-AGAGTTTGATCCTGGCTCAG-3) and rP2 (5-ACGGCTACCTTGTTACGACTT-3) (Weisburg et al. 1991). The PCR reaction mixture (100 μl) contained 5 U of Taq DNA polymerase (Roche, Mannheim, Germany), 100 ng of template, 10 mM Tris-HCl (pH 8.0), 50 mM KCl, 1.5 mM MgCl2, 250 μM dNTP and 0.4 μM of each primer. The mixture was first incubated for 5 min at 95 °C; then, 35 cycles were performed as follows: 4 min at 95 °C, 1 min at 58 °C and 2 1 min at 72 °C. The amplified fragments were purified from an 0.8 % agarose gel using a gel extraction kit (iNtRON Biotechnology, Seongnam, Korea) and sequenced by Cosmogenetech Sequencing Service (Cosmogenetech, Seoul, Korea). The 16S rRNA sequence analysis was performed using BLAST network services at the National Center for Biotechnology Information (NCBI). A phylogenetic tree was constructed with the neighbor-joining method using Molecular Evolutionary Genetics Analysis (MEGA) version 5.1 software package after performing multiple alignment of the sequences using the CLUSTAL W program (Thompson et al. 1997). Bootstrap analysis with 1,000 replicates was conducted to evaluate the stability of the groups in the neighbor-joining tree, using the same program (Felsenstein, 1985). References Bowman JP, Cavanagh J, Austin JJ, Sanderson K (1996) Novel Psychrobacter species from Antarctic ornithogenic soils. Int J Syst Evol Microbiol 46:841–848 Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791 Gerhardt P (1994) Methods for General and Molecular Bacteriology. Washington, DC: American Society for Microbiology Kim HS, Sang MK, Jung HW, Jeun YC, Myung IS, Kim KD (2012) Identification and characterization of Chryseobacterium wanjuense strain KJ9C8 as a biocontrol agent against Phytophthora blight of pepper. Crop Prot 32:129–137 Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882 Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703 2