Review of Atomic and Ionic structure:
advertisement
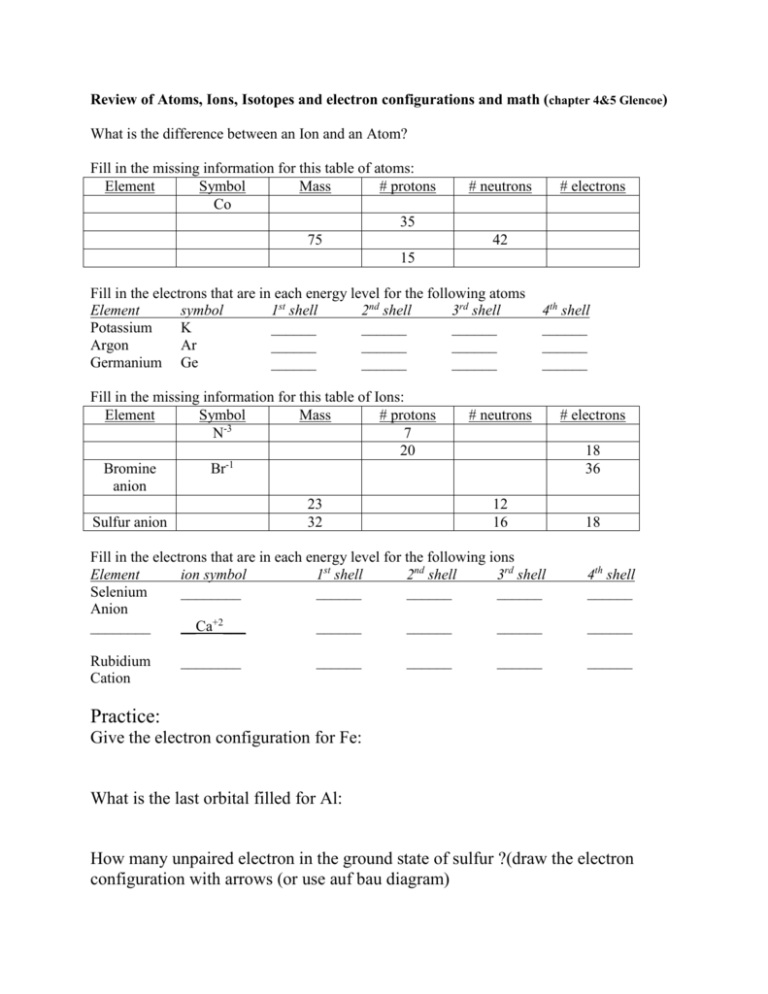
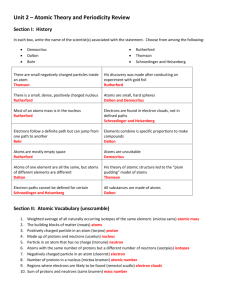
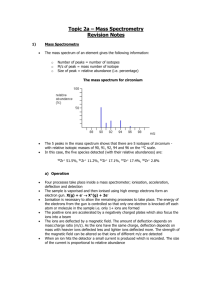
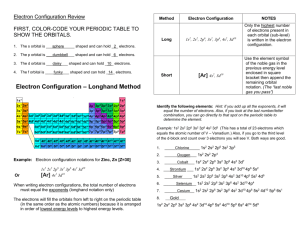
Review of Atoms, Ions, Isotopes and electron configurations and math (chapter 4&5 Glencoe) What is the difference between an Ion and an Atom? Fill in the missing information for this table of atoms: Element Symbol Mass # protons Co 35 75 15 # neutrons # electrons 42 Fill in the electrons that are in each energy level for the following atoms Element symbol 1st shell 2nd shell 3rd shell Potassium K ______ ______ ______ Argon Ar ______ ______ ______ Germanium Ge ______ ______ ______ Fill in the missing information for this table of Ions: Element Symbol Mass # protons -3 N 7 20 Bromine Br-1 anion 23 Sulfur anion 32 4th shell ______ ______ ______ # neutrons 18 36 12 16 Fill in the electrons that are in each energy level for the following ions Element ion symbol 1st shell 2nd shell 3rd shell Selenium ________ ______ ______ ______ Anion ________ __Ca+2___ ______ ______ ______ Rubidium Cation ________ ______ ______ # electrons ______ 18 4th shell ______ ______ ______ Practice: Give the electron configuration for Fe: What is the last orbital filled for Al: How many unpaired electron in the ground state of sulfur ?(draw the electron configuration with arrows (or use auf bau diagram) Which orbital represents higher energy 7s or 4d? Name that element: (and indicate if excited or in ground state) 1s2 2s2 2p6 3s2 3p6 4s2 3d8 1s2 2s1 2p4 1s2 2s2 2p6 3s2 3p6 [Ar]4s2 3d2 1s2 2s2 2p6 3s2 Which electron configuration listed above would also represent the ion Ca+2 What is the frequency of a yellow line of wavelength 550nm (550 x 10-9 m)? What is the energy of a 101.2 FM radio wave? (= 101.2 x 106 Hz) Which has greater energy a yellow light or a green light? Element X has two known isotopes: X-316 = 84.50% and X-320 = 15.50% What is it’s average atomic mass (like the M&M lab) ANSWERS What is the difference between an Ion and an Atom? Atoms are neutral (just as many protons as electrons; Ions have a net charge and are called cations or anions Fill in the missing information for this table of atoms: Element Symbol Mass # protons cobalt Co 59 27 Bromine Br 80 35 Arsenic As 75 33 Phosphorus P 31 15 30 # neutrons 32 45 42 16 15 Fill in the electrons that are in each energy level for the following atoms Element symbol 1st shell 2nd shell 3rd shell Potassium K ___2___ ___8___ ___8___ Argon Ar __2____ ___8___ ___8___ Germanium Ge ___2___ ___8___ ___18___ Fill in the missing information for this table of Ions: Element Symbol Mass # protons -3 Nitrogen N 14 7 anion Calcium Ca+2 40 20 cation Bromine Br-1 80 35 anion Sodium Na+1 23 11 cation Sulfur anion S-2 32 16 # electrons 27 35 33 16 4th shell ___1___ ______ ___4___ # neutrons 7 # electrons 10 20 18 45 36 12 16 18 Fill in the electrons that are in each energy level for the following ions Element ion symbol 1st shell 2nd shell 3rd shell 4th shell Selenium ___Se-2___ ___2___ __8____ ___18___ ___8___ _Calcium_ __Ca+2__ ___2___ __8____ ___8___ __0____ Cation Rubidium ___Rb+1___ ___2___ ___8___ __18____ __8____ Practice: Give the electron configuration for Fe: 1s2 2s2 2p6 3s2 3p6 4s2 3d6 What is the last orbital filled for Al: 1s2 2s2 2p6 3s2 3p1 Last electron goes into 3p (but last COMPLETELY filled is the 3s) How many unpaired electron in the ground state of sulfur 1s2 2s2 2p6 3s2 3p4 ; the 3p orbital looks like up down up up as arrows so… 2 unpaired Which orbital represents higher energy 7s or 4d ? 7s Name that element: (and indicate if excited or in ground state) 1s2 2s2 2p6 3s2 3p6 4s2 3d8 1s2 2s1 2p4 1s2 2s2 2p6 3s2 3p6 ground state Nickel excited nitrogen ground state argon [Ar]4s2 3d2 ground state Titanium 1s2 2s2 2p6 3s2 ground state Magnesium Which electron configuration listed above would also represent the ion Ca+2 1s2 2s2 2p6 3s2 3p6 ground state argon What is the frequency of a yellow line of wavelength 550nm F = c/ ; f= 3.0 x 108 / 550 x 10-9 = 5.45 x 1014 Hz What is the energy of a 101.2 FM radio wave? (E = h f ; h = 6.6 x 10-34 J s) E = 6.6 x 10-34 J s x 101.2 x 106 s-1 = 6.8 x 10-26 J Which has greater energy a yellow light or a green light? Green light it has a shorter wavelength and higher frequency so more energy Element X has two know isotopes: X-316 = 84.50% and X-320 = 15.50% What is it’s average atomic mass (like the M&M lab) 84.5 % of 316 + 15.5 % of 320 = 267.02 + 49.60 = 316.62