Electron Configurations and Orbital Diagrams KEY
advertisement
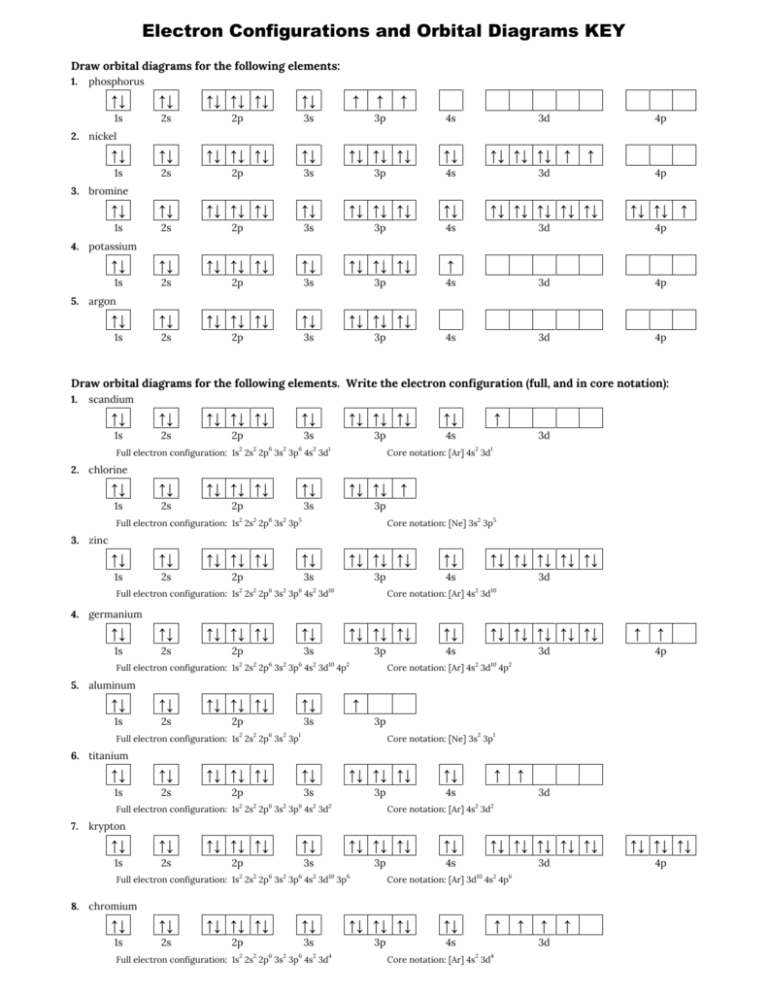
Electron Configurations and Orbital Diagrams KEY Draw orbital diagrams for the following elements: 1. phosphorus ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ 1s 2s 2p 3s ↑ 3p ↑ ↑ 4s ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ 1s 2s 2p 3s 3p 4s 3d 4p ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑ 1s 2s 2p 3s 3p 4s 3d 4p ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑ 1s 2s 2p 3s 3p 4s 3d 4p ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ 1s 2s 2p 3s 3p 4s 3d 4p 3d 4p 2. nickel ↑↓ ↑↓ ↑↓ ↑ ↑ 3. bromine 4. potassium 5. argon Draw orbital diagrams for the following elements. Write the electron configuration (full, and in core notation): 1. scandium ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ 1s 2s 2p 3s 3p 4s Full electron configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d1 ↑ 3d Core notation: [Ar] 4s2 3d1 2. chlorine ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑ 1s 2s 2p 3s 3p Full electron configuration: 1s2 2s2 2p6 3s2 3p5 Core notation: [Ne] 3s2 3p5 3. zinc ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ 1s 2s 2p 3s 3p 4s 2 2 6 2 6 2 Full electron configuration: 1s 2s 2p 3s 3p 4s 3d 10 ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ 3d 2 Core notation: [Ar] 4s 3d 10 4. germanium ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ 1s 2s 2p 3s 3p 4s 3d Full electron configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2 ↑ ↑ 4p Core notation: [Ar] 4s2 3d10 4p2 5. aluminum ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ 1s 2s 2p 3s ↑ 3p Full electron configuration: 1s2 2s2 2p6 3s2 3p1 Core notation: [Ne] 3s2 3p1 6. titanium ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ 1s 2s 2p 3s 3p 4s 2 2 6 2 6 2 Full electron configuration: 1s 2s 2p 3s 3p 4s 3d 7. 2 ↑ ↑ 3d 2 Core notation: [Ar] 4s 3d 2 krypton ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ 1s 2s 2p 3s 3p 4s 3d 4p 2 2 6 2 6 2 10 Full electron configuration: 1s 2s 2p 3s 3p 4s 3d 3p 6 10 2 Core notation: [Ar] 3d 4s 4p 6 8. chromium ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ 1s 2s 2p 3s 3p 4s Full electron configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d4 ↑ Core notation: [Ar] 4s2 3d4 ↑ ↑ 3d ↑ Write the electron configuration (full, and in core notation) for the following ions: Br-1 1. 13. Cu+3 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 10 2 6 [Kr], [Ar] 3d 4s 4p 2. Sr+2 2 6 2 6 10 2 6 2 6 2 6 10 2 6 2 6 2 6 3. Se-2 4. Al+3 1s 2s 2p 2 6 2 6 10 2 6 10 2 6 2 6 10 2 6 10 2 6 2 6 10 2 6 2 6 1s 2s 2p 3s 3p 3d 4s 4p 4d [Kr] 4d 1s2 2s2 2p6 3s2 3p6 3d8 8 [Ar] 3d 10 2 14. Fe+3 1s 2s 2p 3s 3p 3d 4s 4p 4d 10 [Kr] 4d 1s2 2s2 2p6 3s2 3p6 3d5 [Ar] 3d5 2 15. Mn+2 1s 2s 2p 3s 3p 3d [Ar] 3d10 2 2 2 6 6 6. As-3 1s2 2s2 2p6 3s2 3p6 3d104s2 [Ar] 4s2 3d10 2 6 5 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f14 5s2 5p6 5d4 6s1 [Xe] 4f14 5d4 6s1 11. Pt+4 2 6 16. W+1 1s 2s 2p 1s 2s 2p 3s 3p 3d 6 [Ar] 3d 2 1s 2s 2p 3s 3p 3d 5 [Ar] 3d 10. O-2 5. Fe+2 2 2 9. Zn+2 1s 2s 2p 3s 3p 3d 4s 4p 10 2 6 [Kr], [Ar] 3d 4s 4p 2 Sn+4 8. Ag+1 2 1s 2s 2p 3s 3p 3d 4s 4p [Kr], [Ar] 3d10 4s2 4p6 2 7. 2 6 10 2 6 10 14 2 6 1s 2s 2p 3s 3p 3d 4s 4p 4d 4f 5s 5p 5d 14 6 [Xe] 4f 5d 12. Zr+2 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d2 [Kr] 4d2 6 17. Mo+3 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d3 3 [Kr] 4d 18. Pb+2 2 2 6 2 6 10 2 6 10 14 2 6 10 Answer the following questions: 1. Describe the two differences between a 2px orbital and a 3py orbital. The 2px orbital lies on the x-axis. The 3py orbital lies on the y-axis and is larger than the 2px orbital. 2. The lobes of a p orbital disappear at the nucleus. What does this tell us about electrons in p orbitals? The probability of finding an electron at the nucleus is 0 (you will never find an electron in the nucleus). 3. The electron configuration for phosphorus, written in core notation, is [Ne] 3s2 3p3. What two things does Hund’s rule tell us about the three electrons in the 3p sublevel? The 3p electrons are in three different orbitals and have the same spin. 4. Use the periodic table to identify the neutral atoms having the following electron configurations: Electron Configuration [Ne] 3s 2 2 [Ar] 4s 3d Element magnesium 5 [Kr] 5s2 4d10 5p3 2 1s 2s 2p 3s 3p 3d 4s 4p 4d 4f 5s 5p 5d 6s [Xe] 4f14 5d10 6s2 manganese antimony 5. Consider the following ions: N–3, O–2, F–1, Na+1, Mg +2, and Al+3. a. How many electrons are present in each ion? 10 b. Write a single electron configuration representing all the ions. 1s2 2s2 2p6 c. Which neutral atom possesses this electron configuration? Neon