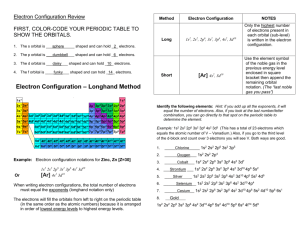
Energy Sublevels - Glasgow Independent Schools
Energy Levels of Electrons
The quantum-mechanical model of the atom also predicts discrete principal energy levels (n) within the atom.
Principal Quantum Numbers seated in each sublevel?
( n ) indicate the relative sizes and energies of atomic orbitals.
Orbitals
Each orbital can contain a maximum
of two electrons in any orbital.
How many electrons can be held in any level?
Use the following formula to calculate total level electrons.
2n 2
Heisenberg Uncertainty
Principle states that it is fundamentally impossible to know precisely both the velocity and position of a particle at the same time.
Energy Sublevels (Shapes)
Energy Sublevels
How many orbitals are in each of the following sublevels?
Sublevel Orbitals Max # Electrons
s 1 only 2 p 3 6 d 5 10 f 7 14
aufbau principle
states that each electron occupies the lowest enerrgy orbital available.
pg. 135 aufbau principle
s p d f
Energy Sublevels to which there are four types
a quick
Sublevel
Diagram
MUST commit this to Memory
&
Follow as you complete
Electron
Config.
Copy the following diagram
Energy Sublevels of Neon
From Orbital Notation to
Electron Configuration Notation
1
H ___
1s
1s
1 Superscript for the total # of electrons
1s
2
2
He
___
1s
3
Li
___
1s
___
2s
1s
2 2s
1
From Orbital Notation to
Electron Configuration Notation
4
Be
___
1s
___
2s
1s
2
2s
2
5
B
___
1s
___
2s
___
2px
___ ___
2py 2pz
1s
2
2s
2
2p
1
6
C
___
1s
___
2s
___
2px
___ ___
2py 2pz
1s
2
2s
2
2p
2
Practice Electron Config
Notation
Your assignment is to write the
Electron Configuration Notation for
Nitrogen through Magnesium
Electron Configuration Notation
7
8
N
O
1s
2
2s
2
2p
3
11
Na 1s
2
2s
2
2p
6
3s
1
1s
2
2s
2
2p
4
12
Mg 1s
2
2s
2
2p
6
3s
2
1s
2
2s
2
2p
5
9
F
10
Ne 1s
2
2s
2
2p
6
Can You Recognize Them??
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 3
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2
4d 5
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 8
Can You Recognize Them??
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 3 As
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2
4d 5 Mo
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 Sn
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 8
Pt