Chapter 4 Electron Configurations
advertisement

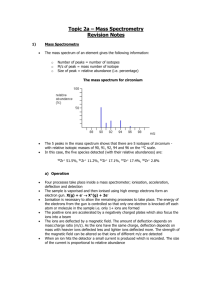
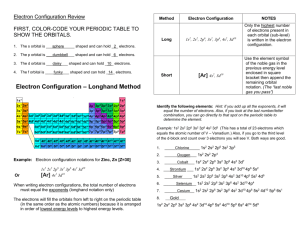
Electron Configurations What is an e- configuration ? • We want to be able to create an econfiguration in order to know where electrons will be found. This will help us with bonding. • It is also helpful in seeing how atoms are stable and how they create compounds. Nature likes stability !! • A common idea throughout Science and not just Chemistry is that nature likes to be in the most stable situation possible. • When given a choice, things will adjust to be in the most stable situation possible. • For example, an object falls when we let go of it because it wants to be in a lower, more stable situation. • Sports gives us many examples. More Stability notes • The lower center of gravity is going to result in a higher stability. • In sports like soccer and basketball, the players on defense want to get low so that they can change directions easily. Notice the player in blue playing defense. More notes on stability • In US football, the linemen get low so that they are more stable and don’t get knocked down by the linemen on the other team. In car racing, the cars are built low to the ground so they are more stable around curves. How do we apply this idea ? • What we need to know is the order of the orbitals from lowest energy to highest energy. • If we know this, we can start placing the electrons where they need to be. • If only we had an easy way, electron configuration life would be so much easier. • Yes, minions, wait for it…. The Diagonal Rule Your best friend when doing e- configurations 1s 2s 3s 4s 5s 6s 7s 2p 3p 4p 5p 6p 7p 3d 4d 4f 5d 5f 6d 6f 7d The arrows start with 1s which is the orbital with lowest energy. We simply want to follow the arrows and that gives us the order of orbitals from lowest energy to highest energy. EUREKA !! • The chart can be made as big as possible, however this size is OK for any atom currently known which is up to about element 118. • If a larger chart is needed (perhaps for some hypothetical atom in extra credit), there is a larger sheet on the class website. • www.scramlinged.com Electron Configuration • ELECTRONS ALWAYS GO INTO THE ORBITAL WITH THE LOWEST POSSIBLE ENERGY !! • Refer to the diagonal rule when doing this. • Remember our little phrase. s 1 p 3 d 5 f 7 Aufbau Principle • Aufbau is German for ‘to build up’ • Electrons are added one at a time to the lowest energy orbitals available until all the electrons of the atom have been accounted for. • The number of electrons in a neutral atom equals the atomic number of the element. Pauli Exclusion Principle • An orbital can hold a maximum of 2 electrons, but they must be of opposite spins. • A lone electron is unpaired while 2 opposite electrons together is paired. Hund’s Rule • Electrons are first placed into 2p orbitals keeping them unpaired for as long as possible. • In other words, to be more stable, an electron will enter an empty orbital before it will go into an orbital with an electron already in it. Let’s get started # 1 • With your diagonal rule by your side, let’s start with a small atom – Nitrogen. • We first determine how many electrons we need. Nitrogen has an atomic number of 7 (# of protons) and since this is an atom, the # of electrons = the # of protons. So we need 7 electrons. • The diagonal rule says we start with the orbital with the lowest energy; 1s Let’s get started # 1 • Following the diagonal rule gives us the following electron configuration for Nitrogen. • • • • 1s2 2s2 2p3 For a final result of: 1s2 2s2 2p3 Example # 2 • • • • Now try Nickel. We see it has 28 electrons. 1s2 2s2 2p6 3s2 3p6 4s2 3d8 However, we need to rearrange the orbitals for reasons we will take later. • We rearrange in order of Principal Quantum Number as follows: • 1s2 2s2 2p6 3s2 3p6 3d8 4s2 Example # 3 • Osmium has 76 electrons. • 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d6 • Now let’s rearrange • 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f14 5s2 5p6 5d6 6s2 Exceptions to the Aufbau Principle • Not every element exactly follows the aufbau principle. For example: Chromium and Copper Through experimentation, it has been found that sets of orbitals that are either filled or half-filled are more stable and as we learned, NATURE LOVES TO BE AS STABLE AS POSSIBLE. • Let’s look at Chromium now, shall we ? Chromium • 1s2 2s2 2p6 3s2 3p6 3d4 4s2 is what we would expect, however it looks like this: • 1s2 2s2 2p6 3s2 3p6 3d5 4s1 • Notice that the d – orbitals which has 5 orbitals and can hold 10 electrons is half-filled so it is more stable. • It is just how it looks – an electron from the s – orbital moves to the d – orbitals. • On a personal note, I am very fond of chromium since my artificial knee is made of chromium stainless steel. It feels great - thanks for asking ! EXTRA CREDIT • The first three people who gives me the correct electron configuration for the hypothetical atom that has 350 electrons will receive a bounty of extra credit points. • However, I will not inform anyone of how many I have received so be forewarned. • Speaking of Bounty, who was the captain of the HMS Bounty which was subjected to a mutiny in the year 1789 ? Who led the mutiny ? More extra credit for the first person who gives me the answers on a piece of paper with their name on it. Electron configurations of ions • There are two types of ions. • CATIONS are positive ions like Sodium (Na+1) and Magnesium (Mg+2). • ANIONS are negative ions like Bromide (Br -1) and Sulfide (S-2). • For both, we start by writing out the econfiguration of the atom, ignoring the charge (for now). e- configurations of cations • Let’s look at the Barium ion (Ba+2). • It looks like this: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 Rearranged: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2 • Since it has a charge of + 2 we need to REMOVE 2 electrons. We get: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 e- configurations of cations • Let’s look at the Iron ion (Fe+3). • It looks like this: 1s2 2s2 2p6 3s2 3p6 4s2 3d6 Rearranged: 1s2 2s2 2p6 3s2 3p6 3d6 4s2 • Since it has a charge of + 3 we need to REMOVE 3 electrons. We get: 1s2 2s2 2p6 3s2 3p6 3d5 e- configurations of anions • Now let’s look at Bromide ion (Br-1). • Here is the electron configuration of the atom after it is rearranged. • 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p5 • Since it is a -1 charge we need to add an electron. • So the new configuration is: – 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 • Anions are easier to do than cations.