HomeworkCh_14Answers
advertisement

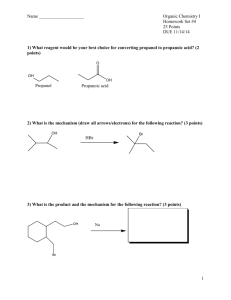
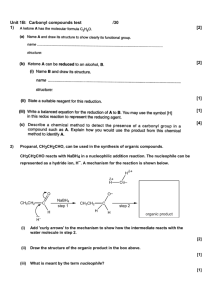
Chapter 14 1. a. What is organic chemistry? The study of carbon, its properties and reactions. b. What is the goal of natural products chemist? Natural products chemists study compounds produced by plants that have biological activity. 2. Which of the following are organic compounds? a. NH3, not organic, doesn’t contain carbon b. C6H12O6, organic c. NaCl, not organic, doesn’t contain carbon d. CH2Cl2, organic 3. Classify each of the following as a natural product, parent compound, analogue, or derivative. Note that there may be more than one description for some of these compounds. a. Salicin, natural product, parent compound of aspirin b. Acetylsalicyclic acid (Aspirin), analoque, derivative of salicin c. Taxol, natural product, parent compound d. Methyl salicylate (oil of wintergreen), natural product 4. There are two isomers corresponding to the chemical formula C2H6O. One is an alcohol, and the other is an ether. a. Draw each compound. CH3CH2OH (alcohol) and CH3OCH3 (ether) b. Which compound would you expect to be more water soluble, and why? Alcohols are more soluble than ethers due to their polarity. c. Which compound would you expect to have the higher boiling point, and why? The alcohol, ethers are non-polar and thus similar to hydrocarbons, which have low boiling points. 5. Draw 3-carbon structures with the following functional groups, note a functional group may be part of the chain. a. An alcohol, CH3CH2CH2OH b. A carboxylic acid, CH3CH2CO2H c. An aldehyde, CH3CH2HC=O d. An amine, CH3CH2CH2NH2