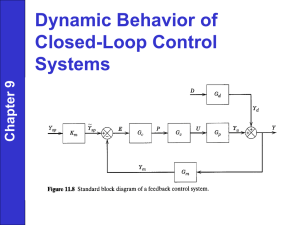
ONLINE APPENDIX TISSUE HARVEST, PREPARATION, AND
advertisement

ONLINE APPENDIX TISSUE HARVEST, PREPARATION, AND ANALYSIS. At the completion of a study period, each animal was euthanized using Fatal-Plus solution (phenobarbital 390 mg/ml, propylene glycol 0.01 mg/ml, ethyl alcohol 0.29 mg/ml, benzyl alcohol 0.2 mg/ml) 1 ml/10 lbs. Tricuspid valve tissue was collected en bloc to include the papillary muscles as well as both atrial and ventricular tissue around the native annulus. Explanted valve tissue was inspected for any gross thrombus, leaflet defects, or calcification. Each valve was prepared in Dulbecco's phosphate buffered saline (Hyclone, GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom) and supplemented with protease inhibitor (1:100, Sigma-Aldrich Co., LLC, St. Louis, Missouri) until it was divided into 3 pieces, each with a papillary attachment: 1 fresh piece was used for biomechanical testing, 1 fixed piece for histopathologic evaluation, and the third piece was preserved for electron microscopy, as previously described (1, 2). The same leaflet was used for the same experiments each time to maintain consistency. Leaflets were sectioned at various portions of the valve including annulus, papillary attachments, and commissure; however, all the comparisons were made between the same areas of the valve leaflet. Histologic evaluation included hematoxylin and eosin (H&E) to show cellular infiltration, alizarin red to show mineralization, and Movat’s pentachrome to show ECM organization. Immunohistochemistry was performed using antibodies directed against CD31 (1:50; Abcam plc, Cambridge, United Kingdom) to identify endothelial cells, CD 68 (1:100, Dako, Carpinteria, California) to identify macrophages, fetal liver kinase 1/vascular endothelial growth factor receptor 2 (FLK/VEGFR2; 1:500; 55B11) to identify cell signaling, and alpha smooth muscle actin (SMA, 1:500, Sigma) to identify resident valve interstitial cells (VIC). Ultrastructure analysis was performed using a Hitachi 7600 (Hitachi High Technologies America, Inc., Schaumburg, Illinois) electron microscope. In order to visualize collagens and elastic fibers, sections were counter stained with aqueous solutions of 5% tannic acid followed by 1% uranyl acetate, and then counterstained with lead citrate, as described previously (3). Mechanical testing of valve tissue was performed using micropipette aspiration as previously described (2). This was done at 3 distinct points on the leaflet (close to annulus, equidistance from annulus and papillary attachment and close to papillary attachment). All these points were homogenous across the biomechanical experiments. The valve surface was accessed from the ventricular side using a micromanipulator and glass micropipette drawn to an inner diameter of 100 μm; upon contact, aspiration pressure was applied in a stepwise manner (4). Videos of tissue aspiration were recorded and the aspiration length and radius were measured. Linear regression was used to curve fit the data. Young’s modulus (E) as a measure of tissue stiffness was determined using a half-space model equation (5,6). One-way analysis of variance tests were used to determine effects on Young’s modulus by valve type (SIS-ECM, NV), valve leaflet region (proximal, distal), and age (3 months post-implant, 8 months post-implant). References: 1. Hinton RB Jr, Lincoln J, Deutsch GH, et al. Extracellular matric remodeling and organization in developing and diseased aortic valves. Circ Res 2006;98:1431-8. 2. Krishnamurthy VK, Guilak F, Narmoneva DA, Hinton RB. Regional structure-function relationships in mouse aortic valve tissue. J Biomech 2011;44:77-83. 3. Hinton RB, Adelman-Brown J, Witt S, et al. Elastin haploinsufficiency results in progressive aortic valve malformation and latent valve disease in a mouse model. Circ Res 2010;107:549-57. 4. Jones WR, Ting-Beall HP, Lee GM, Kelley SS, Hochmuth RM, Guilak F. Alterations in the Young’s modulus and volumetric properties of chondrocytes isolated from normal and osteoarthritic human cartilage. J Biomech 1999;32:119-27. 5. Guilak F, Alexopoulos LG, Haider MA, Ting-Beall HP, Setton LA. Zonal uniformity in mechanical properties of the chondrocyte pericellular matrix: micropipette aspiration of canine chondrons isolated by cartilage homogenization. Ann Biomed Eng 2005;33:131218. 6. Theret DP, Levesque MJ, Sato M, Nerem RM, Wheeler LT. The application of a homogenous half-space model in the analysis of endothelial cell micropipette measurements. J Biomec Eng 1988;110:190-9.