Scientist
advertisement
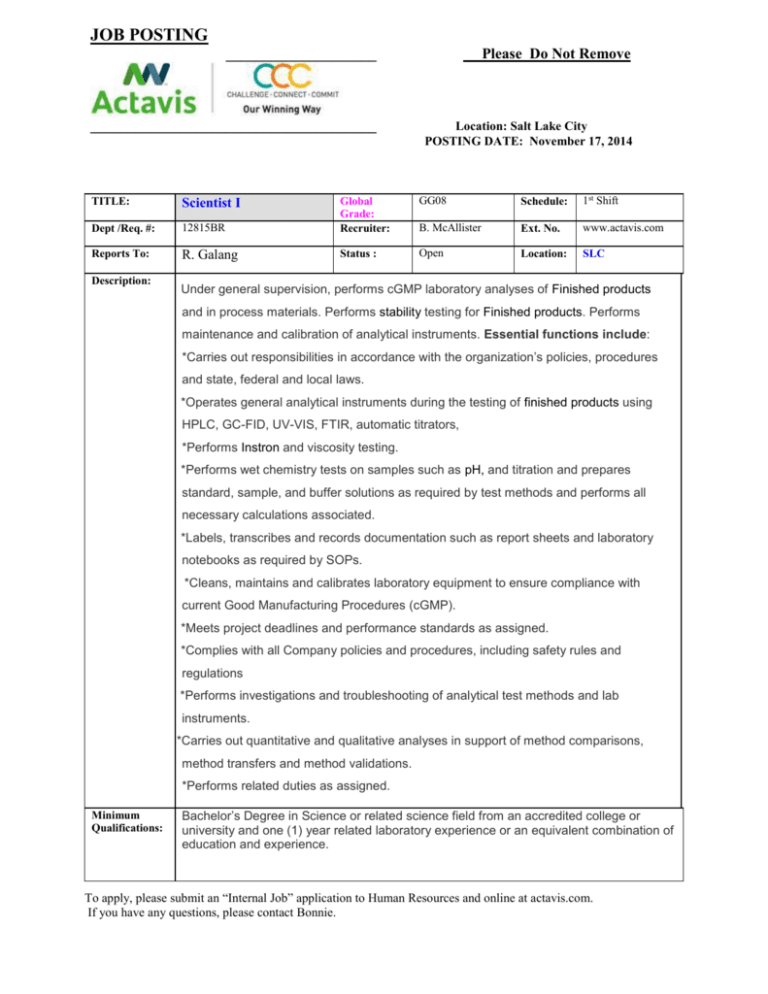
JOB POSTING ____________________ Please Do Not Remove ______________________________________ Location: Salt Lake City POSTING DATE: November 17, 2014 GG08 Schedule: 1st Shift 12815BR Global Grade: Recruiter: B. McAllister Ext. No. www.actavis.com R. Galang Status : Open Location: SLC TITLE: Scientist I Dept /Req. #: Reports To: Description: Under general supervision, performs cGMP laboratory analyses of Finished products and in process materials. Performs stability testing for Finished products. Performs maintenance and calibration of analytical instruments. Essential functions include: · *Carries out responsibilities in accordance with the organization’s policies, procedures and state, federal and local laws. · *Operates general analytical instruments during the testing of finished products using HPLC, GC-FID, UV-VIS, FTIR, automatic titrators, *Performs Instron and viscosity testing. · *Performs wet chemistry tests on samples such as pH, and titration and prepares standard, sample, and buffer solutions as required by test methods and performs all necessary calculations associated. · *Labels, transcribes and records documentation such as report sheets and laboratory notebooks as required by SOPs. · *Cleans, maintains and calibrates laboratory equipment to ensure compliance with current Good Manufacturing Procedures (cGMP). · *Meets project deadlines and performance standards as assigned. · *Complies with all Company policies and procedures, including safety rules and regulations *Performs investigations and troubleshooting of analytical test methods and lab instruments. .*C*Carries out quantitative and qualitative analyses in support of method comparisons, method transfers and method validations. *Performs related duties as assigned. Minimum Qualifications: Bachelor’s Degree in Science or related science field from an accredited college or university and one (1) year related laboratory experience or an equivalent combination of education and experience. To apply, please submit an “Internal Job” application to Human Resources and online at actavis.com. If you have any questions, please contact Bonnie. JOB POSTING ____________________ Please Do Not Remove ______________________________________ Location: Salt Lake City POSTING DATE: November 17, 2014 Experience and Skills: •Usage of GC and HPLC chromatographic equipment, as well as UV/VIS, and FTIR operation methods and techniques. •Business, scientific and personal computer hardware and software applications. •Business English usage, spelling, grammar and punctuation. •Wet Chemistry and Chemistry related to sampling methods, quality control systems, analysis and documentation practices and procedures. •Knowledge of or ability to learn FDA, cGLP, cGMP, and SOP or other applicable regulatory and safety compliance guidelines. •Performing testing accurately and precisely. •Responding to routine inquiries from management, employees and regulatory agencies. •Communicating clearly and concisely, both orally and in writing. •Managing multiple projects, duties and assignments. •Establishing and maintaining cooperative working relationships with others. EOE Minorities/Females/Protected Veterans/Disabled To apply, please submit an “Internal Job” application to Human Resources and online at actavis.com. If you have any questions, please contact Bonnie.