Solubility Worksheet
advertisement
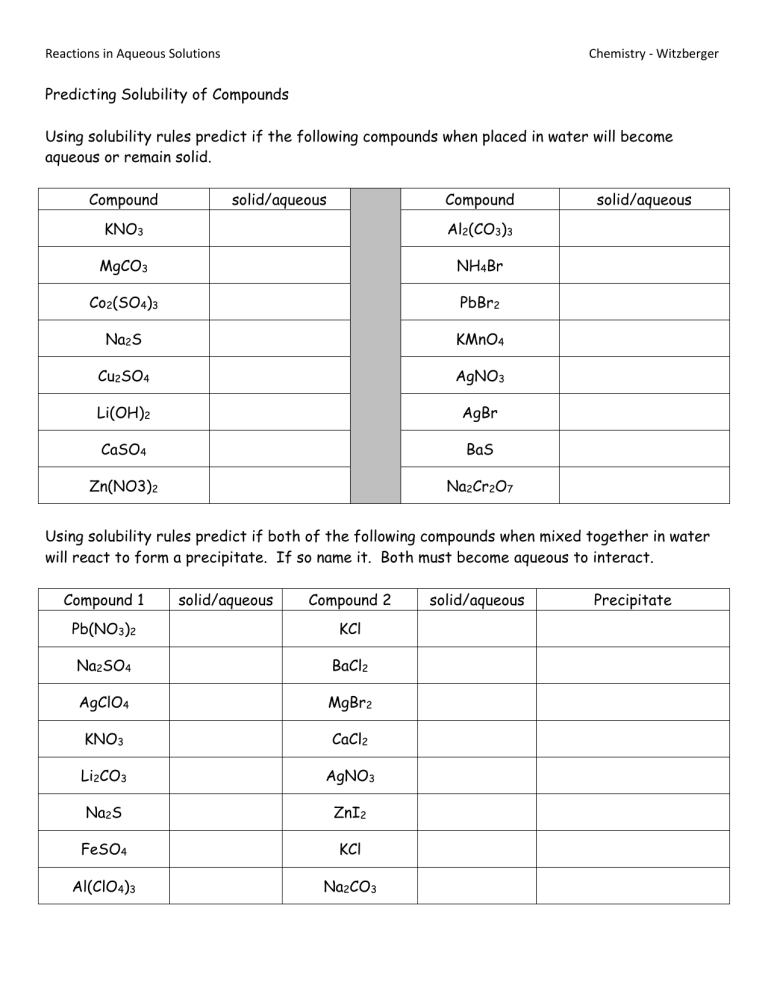
Reactions in Aqueous Solutions Chemistry - Witzberger Predicting Solubility of Compounds Using solubility rules predict if the following compounds when placed in water will become aqueous or remain solid. Compound solid/aqueous Compound KNO3 Al2(CO3)3 MgCO3 NH4Br Co2(SO4)3 PbBr2 Na2S KMnO4 Cu2SO4 AgNO3 Li(OH)2 AgBr CaSO4 BaS Zn(NO3)2 Na2Cr2O7 solid/aqueous Using solubility rules predict if both of the following compounds when mixed together in water will react to form a precipitate. If so name it. Both must become aqueous to interact. Compound 1 solid/aqueous Compound 2 Pb(NO3)2 KCl Na2SO4 BaCl2 AgClO4 MgBr2 KNO3 CaCl2 Li2CO3 AgNO3 Na2S ZnI2 FeSO4 KCl Al(ClO4)3 Na2CO3 solid/aqueous Precipitate






