5.7 Unique Properties
advertisement

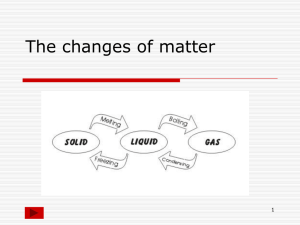
5.7 Unique Properties Your fingerprint is a unique characteristic that distinguishes you from others. Fingerprints are commonly used to identify people. Your fingerprint will remain the same, even if other physical properties--such as your height or weight--change. Characteristic Physical Properties Substances also have unique characteristics that can be used to identify them. A characteristic property is a physical or chemical property that is unique to a particular substance. BEGIN SIDEBAR: - characteristic property: a property that is unique to a substance and that can be used to identify the substance END SIDEBAR. Freezing Point, Melting Point, and Boiling Point The temperatures at which common physical changes like freezing, melting, and boiling take place are characteristic properties of a substance because they do not change. - Freezing point is the temperature at which a substance turns from liquid to solid. The freezing point of a substance is also the same temperature as its melting point. - Melting point is the temperature at which a substance turns from a solid into a liquid. The freezing/melting point of pure water is 0 °C. - Boiling point is the temperature at which a substance forms bubbles of vapour that escape into the air. Pure water boils at 100 °C. Pure Substances Carbon has the highest melting point of all pure substances. It melts at 3550 °C. Helium has the lowest melting point of all pure substances. It melts at -272.2 °C. Tungsten has the highest boiling point. It boils at 5927 °C. Gallium is a metal that is so soft, it can be cut with a knife. Its melting point of 29.8 °C is unusually low for a metal. The temperature of the human body is 37 °C. That means that if you hold gallium in the palm of your hand, as shown in Figure 1, the gallium will melt. Despite its low melting point, gallium has an extremely high boiling point of 2403 °C. The range of temperatures at which gallium is a liquid is the greatest of any pure metallic substance. BEGIN FIGURE CAPTION: Figure 1 The unusual melting and boiling points of gallium make it useful for special high-temperature thermometers. BEGIN PRODUCER’S NOTE: Figure not reproduced. END PRODUCER’S NOTE. END FIGURE CAPTION. PRINT PAGE 191 Mixtures The freezing/melting point of a mixture of substances is different than the freezing/melting point of a pure substance. For example, dissolving another substance into water forms a mixture with a lower freezing point than pure water. For example, a salt and water mixture can have a freezing point as low as -10 °C. This is why salt is used to make icy roads safer to drive on (Figure 2). Spreading salt on the roads allows the saltwater solution to remain liquid below 0 °C. Liquid water is safer to drive on than ice. BEGIN FIGURE CAPTION: Figure 2 Icy roads are dangerous. A salt spreader adds salt to icy roads. This creates a mixture with a freezing point lower than 0 °C, making roads safer at low temperatures. BEGIN PRODUCER’S NOTE: Figure not reproduced. END PRODUCER’S NOTE. END FIGURE CAPTION. Liquid water is also important in a car engine. You could use water to cool your car's engine, as long as its temperatures stayed between the freezing and boiling points of water. However, once the temperature drops below 0 °C, the cooling system freezes solid. Adding antifreeze to the water forms a mixture with a lower freezing point, and a higher boiling point, than pure water. This prevents the cooling system from freezing in the winter and "boiling over" in the summer. Density Density is also a characteristic physical property. Density is the mass in a given volume of a substance. Density is expressed as mass per volume, or mass/volume. Each substance has its own characteristic density. For example, the density of pure water is 1 g/mL. This means that 1 mL of pure water has a mass of 1 g. Densities are quite variable. For example, the density of lead is 11.36 g/cm3 while the density of aluminum is only 2.7 g/cm3. Gold has a density of 19.32 g/cm3. BEGIN SIDEBAR: - density: a measure of how much mass is contained in a given volume of a substance; you calculate density by dividing the mass of a sample by its volume END SIDEBAR. Lifejackets The density of the average human is just about the same as that of water. We can sink or float, depending on how much air we hold in our lungs. A lifejacket is very helpful when we are unable to hold enough air in our lungs. An inflatable lifejacket like the one in Figure 3 has a tiny cylinder of compressed carbon dioxide gas attached inside it. When you pull the ripcord, shown on the lower left in the photo, the compressed gas rushes out of the cylinder and inflates the lifejacket. The mass of the jacket does not change, but its volume increases significantly. That means the density of the lifejacket is much lower than the density of the water you are in. This fact helps to keep you safely floating on the water instead of sinking to the bottom. BEGIN FIGURE CAPTION: Figure 3 In Canada, the law requires every boat to carry a personal flotation device (PFD) for each passenger. BEGIN PRODUCER’S NOTE: Figure not reproduced. END PRODUCER’S NOTE. END FIGURE CAPTION. PRINT PAGE 192 BEGIN TEXTBOX: 5.7 Wrap Up - A characteristic property is a property that is unique to a substance. - Characteristic properties can be used to identify a substance. - Freezing point, melting point, boiling point, and density are characteristic physical properties. - Adding another substance to a pure substance produces a mixture with a lower freezing point. BEGIN TEXTBOX: CHECK YOUR LEARNING 1. Explain why the boiling point of water is a characteristic physical property, but the temperature of water is not. K/U, T/I 2. Describe what happens to the freezing point of pure water when salt is added to it. K/U 6. A drinking glass at a crime scene contains a clear, colourless liquid that could be pure water. You are the investigator. Before you send the liquid to a lab for testing, you want to rule out the possibility that the glass contains water. Use what you know about the characteristic properties of water to design a simple test. T/I, C