SI WORKSHEET 19 *Lone pairs not shown unless necessary
SI WORKSHEET 19
*Lone pairs not shown unless necessary*
1.
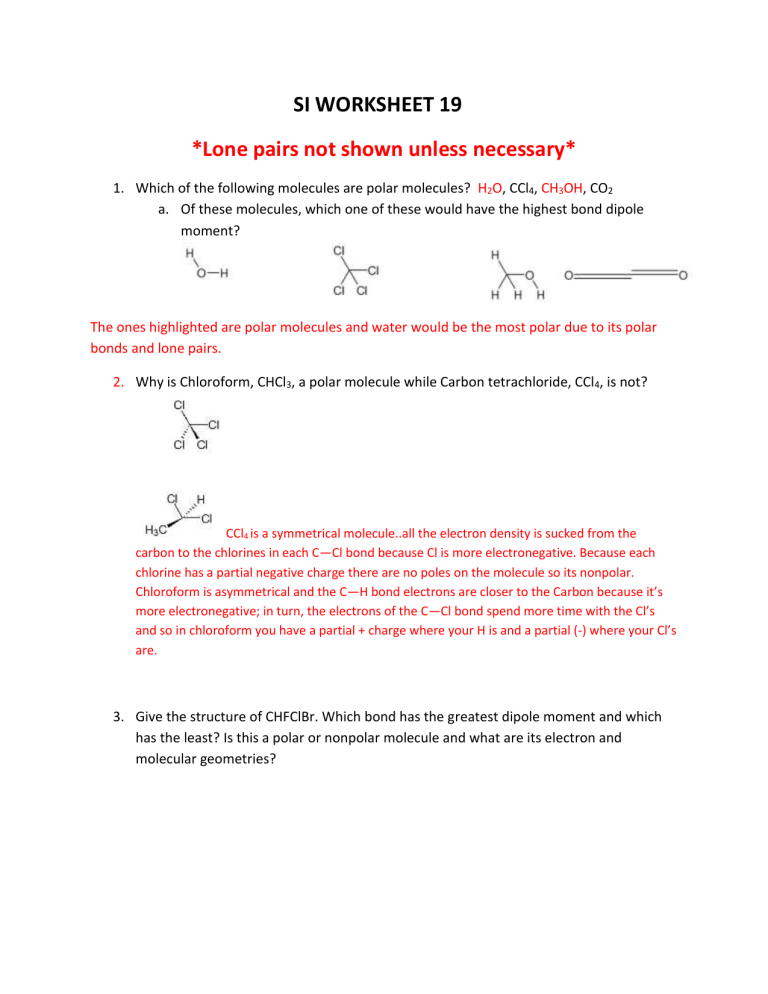
Which of the following molecules are polar molecules? H
2
O , CCl
4
, CH
3
OH , CO
2 a.
Of these molecules, which one of these would have the highest bond dipole moment?
The ones highlighted are polar molecules and water would be the most polar due to its polar bonds and lone pairs.
2.
Why is Chloroform, CHCl
3
, a polar molecule while Carbon tetrachloride, CCl
4
, is not?
CCl
4 is a symmetrical molecule..all the electron density is sucked from the carbon to the chlorines in each C—Cl bond because Cl is more electronegative. Because each chlorine has a partial negative charge there are no poles on the molecule so its nonpolar.
Chloroform is asymmetrical and the C—H bond electrons are closer to the Carbon because it’s more electronegative; in turn, the electrons of the C—Cl bond spend more time with the Cl’s and so in chloroform you have a partial + charge where your H is and a partial (-) where your Cl’s are.
3.
Give the structure of CHFClBr. Which bond has the greatest dipole moment and which has the least? Is this a polar or nonpolar molecule and what are its electron and molecular geometries?
The electronegative difference is greatest in the C—F bond (most polar; greatest dipole) and least in the C—Br bond (least polar, least dipole). Overall this molecule is asymmetrical and is polar due to the C—F and C—Cl bonds having a partial (-) and the C—Br and
C—H having a partial (+) charge.
4.
Acetone, CH
3
COCH
3 is shown to the left, what is the hybridization of the central carbon, how many sigma and pi bonds are present, and is this a nonpolar or polar molecule? What are the geometries around the central carbon ? The central carbon is sp 2 hybridized due to the double bond (trigonal planar geometry). There are 9 sigma bonds and 1 pi bond in this molecule (Don’t forget your methyl groups); this is a polar molecule because the Oxygen end of the molecule is slightly negatively charged and + charge where methyl groups are.
5.
Which of these molecules could participate in hydrogen bonding: H
2
O , NH
3
, CH
4
, CH
2
F
2
?
Hydrogen bonding takes place only when a hydrogen is bound to a very electronegative atom
(F, O, N, and sometimes Cl) and that atom’s lone pairs are attracted to the H’s on other electronegative atoms. No Hydrogen bonding if there are not lone pairs and if the H isn’t bound to the 4 atoms previously mentioned. And yes I just noticed that I added Chlorines instead of
Fluorines to what was to be CH
2
F
2
.
a.
Do molecules that have hydrogen bonding have stronger or weaker melting and boiling points than molecules that do not have such interactions? Stronger
because it takes more energy to make that molecule change states because you have to break the hydrogen bond.
6.
Given NaCl, CO
2
, H
2,
I
2
, and H
2
O: a.
What types of bonds/interactions are part of each compound?
NaCl—ionic bonds
H
2
O—dipole moments, covalent bonds, wan der waal interactions
CO
2
—covalent bond and wan der waal interactions
H
2
— wan der waal interactions
I
2
— wan der waal interactions b.
List these compounds in order of bond strength from strongest to weakest. NaCl,
H
2
O, CO
2
, H
2, and I
2
7.
Which molecule has the largest dipole moment? Order them from least to greatest: PH
3
, NH
3
,
H
2
O, and H
2
S
Water has the largest dipole moment, then ammonia, then Hydrogen sulfide and then Phosphine, dipole moments are based on polarity which is based on electronegativity











