Molecular Polarity Worksheet: Simulation & Concepts
advertisement

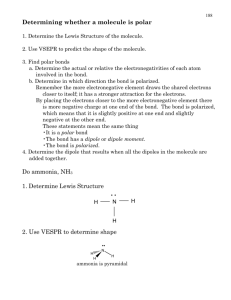
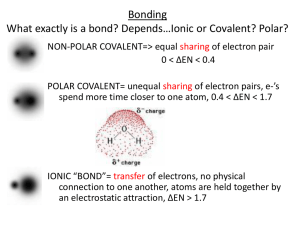
Names ……………………………………………… Molecular polarity Explore the molecular polarity simulation at http://phet.colorado.edu/en/simulation/molecule-polarity Start with the Two Atoms tab. 1. Adjust the electronegativity of atom A and atom B. a) Explain what the bond dipole arrow shows. b) State when the bond dipole arrow points left and when it points right. c) Describe how the bond dipole can be made smaller or larger. d) Describe the relationship between the bond dipole, partial charges and bond character. 2. a) Describe what the electron density is. b) Describe what the electrostatic potential is and how it can change. c) Describe the relationship between electron density and electrostatic potential. 3. Turn on the electric field for a series of different two-atom molecules. Explain why the molecules move or don’t move. Names ……………………………………………… Move on to the Three Atoms tab. 4. Explain how the partial charge on B is affected by the electronegativity of A, B and C. 5. Describe how the molecular dipole is related to the two individual bond dipoles A-B and B-C. 6. Describe the effect of changing the bond angle ABC on the molecular dipole. Work with the Real Molecules tab. 7. Find an example molecule for each of the categories and write a paragraph about each one. (You could also copy paste an image of the molecule.) a) A non-polar molecule with non-polar bonds, b) A non-polar molecule with polar bonds, c) A polar molecule containing 2 or 3 atoms, d) A polar molecule containing 4 or more atoms.