Chemical Bonding Review: Types, Polarity, and VSEPR
advertisement

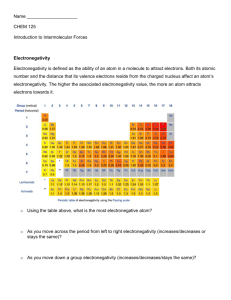
9 Chemical Bonding Review 1. What are the two types of bonds that can form between atoms? 2. What type of bond forms between: A. Two identical atoms B. A metal and a nonmetal 3. What is meant by the term “polar covalent bond”? Give two examples of molecules that would contain polar covalent bond(s): 4. Which of the following molecules would contain an ionic bond? How do you know this? A. CO C. HBr B. CaBr2 D. Cl2 5. Arrange the following atoms based on electronegativity. Place the most electronegative atom on the right and the least electronegative atom on the left. P Al Cl Mg 6. Which of the following bonds would be most polar? Which would be least polar? WHY? A. P-Cl C. N-H B. H-H D. C-F 7. Explain why sulfur “achieves a Noble gas configuration” when it forms an S-2 ion, or forms bonds with two hydrogen atoms as in H2S: 8. Draw Lewis structures and Use the VSEPR model to determine the molecular geometry (3-D shape) of the following molecules. Predict whether the molecule is polar overall. A. CH3Br Polar? Y or N B. BH3 Polar? Y or N C. SiH4 Polar? Y or N 9. Define electronegativity. Describe the pattern of this property based on an element’s location on the Periodic Table. How is electronegativity related to bond polarity? 10. What are “valence” electrons? What is the relationship between valence electrons and chemical bonds? 11. What is VSEPR? 12. How do lone pairs influence or affect the geometry of molecules?